

© National Comprehensive Cancer Network, Inc. 2024, All rights reserved.
NCCN Best Practices Committee
New Information and Survey Results from June 2024
Publication Date: June 26, 2024
Survey data collected from May 28, 2024 – June 11, 2024

New Information and Survey Results from June 2024
1a. Please indicate how many (if any) drugs are currently in short supply at your center?
(N=28)
0 Drugs
11%
1 Drug
14%
2 Drugs
29%
3 Drugs
18%
4 Drugs
11%
5 Drugs
11%
7 Drugs
3%
9 Drugs
3%

New Information and Survey Results from June 2024
1b. Please indicate which (if any) drugs are currently in short supply at your center?
(N=28)
57%
46% 46%
43%
18%
14% 14%
11%
7% 7%
4% 4% 4%
11%
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
Other:
•
BCG Tice
•
Dexrazoxane
•
Docetaxel
•
Doxorubicin HCl Liposome
•
Dronabinol
•
Fludarabine
•
Hydromorphone (injection)
•
Irinotecan
•
Iron Sucrose
•
Liposomal Doxorubin
•
Lorazepam (IV and Injection)
•
Mesna
•
Mitomycin
•
Mitoxantrone
•
Octreotide
•
Vincristine

Yes
75%
No
25%
New Information and Survey Results from June 2024
2. Has your center been made aware of drug shortage issues within community practices in your area (either within
your center’s network or outside)?
(N=28)

Yes, with
mitigation
strategies
enacted
56%
Yes, without
mitigation
strategies
enacted
37%
Not applicable, no
drugs in short
supply
7%
New Information and Survey Results from June 2024
3. For drugs in short supply at your center, is your center able to treat all patients who are currently receiving those
regimens according to the intended dose and schedule?
(N=27)

New Information and Survey Results from June 2024
4. What mitigation strategies is your center using to navigate the drug shortages?
(N=15)*
80%
53%
33% 33%
47%
Implement waste management
strategies
Limit use of current stock Use range minimum for
recommended dose (e.g., Give
4mg/mL does for a range of 4-
5mg/mL dose)
Use range maximum for
recommended treatment interval
(e.g., Schedule treatment every
4th week for a range of 3-4 week
treatment intervals)
Other, please specify
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
*Includes only respondents who answered “Yes, with mitigation strategies enacted” for Question 3.
Other:
•
Dose rounding.
•
Limiting restrictions.
•
Indication prioritization.
•
Reserve supplies for patients on active treatment and curative
intent.
•
Based on expected usage to maintain PAR levels of generic
medications with limited access to treat multiple cycles.
•
Sourcing from alternative wholesalers outside primary
accounts/GPO (still official wholesalers, not gray market).
•
Proactive management of inventory strategy.

Re-prior authorization is needed
when treatment plan is modified
prior to AND during existing
treatment
86%
Re-Prior authorization is
needed when treatment plan
is modified during existing
treatment only
7%
Re-prior authorization is not
required when treatment plans are
modified or interrupted
7%
New Information and Survey Results from June 2024
5. When treatment plans are modified or interrupted due to a drug shortage, which of the below statements are mostly
true for your center?
(N=14)*
*Includes only respondents who answered “Yes, with mitigation strategies enacted” for Question 3.

No
60%
Yes
27%
Not Applicable
13%
New Information and Survey Results from June 2024
6. Has re-obtaining prior-authorizations because of modified treatment plans (due to shortages) resulted in treatment
delay?
(N=15)*
*Includes only respondents who answered “Yes, with mitigation strategies enacted” for Question 3.

No
57%
Yes
43%
New Information and Survey Results from June 2024
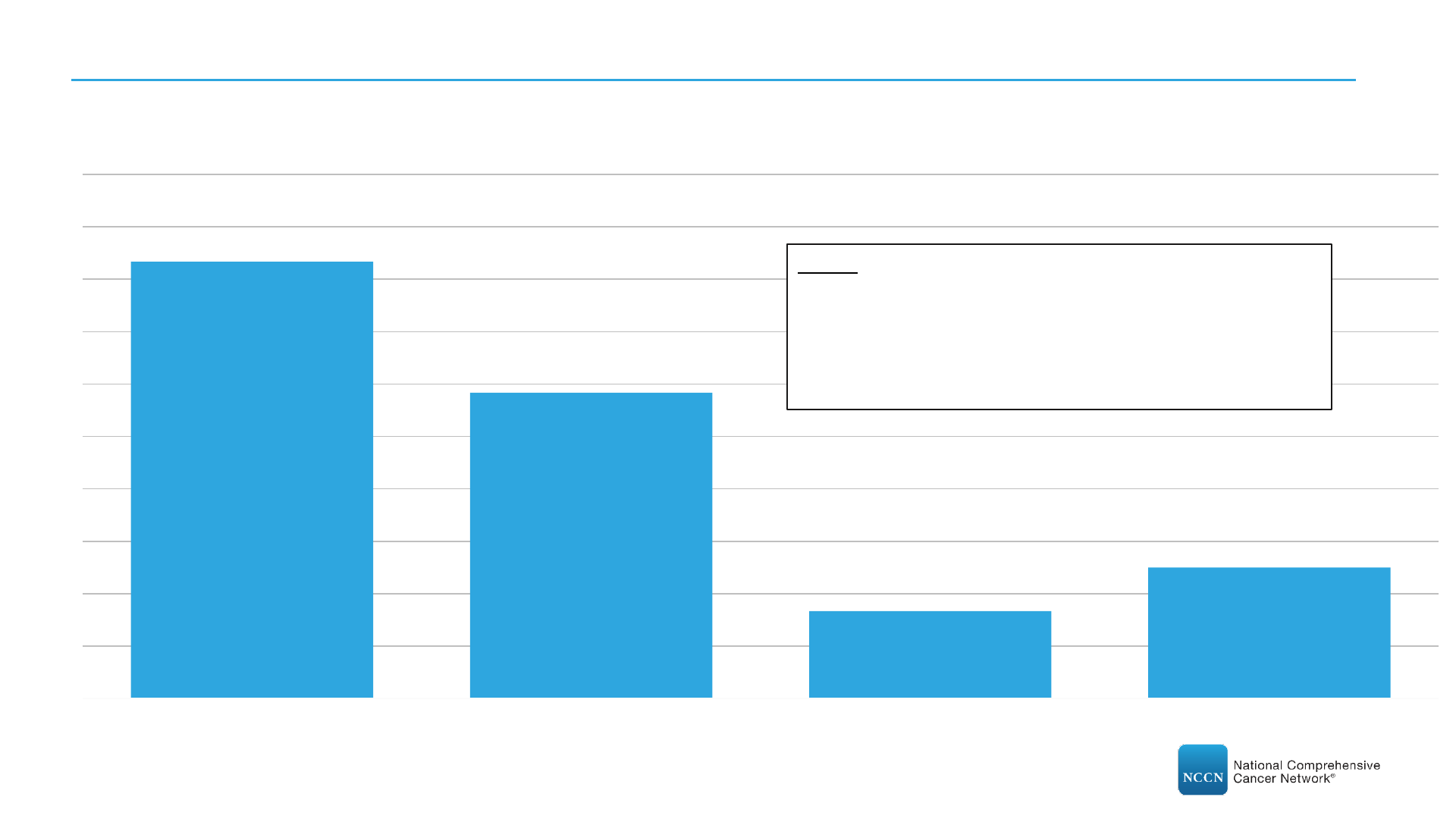
7. Have drug shortages impacted clinical trials at your center?
(N=28)
New Information and Survey Results from June 2024
8. How have drug shortages impacted clinical trials?
(N=12)*
83%
58%
17%
25%
Greater administrative burden Reduction in enrollments Reduction in open trails Other, please specify:
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
*Includes only respondents who answered “Yes” to Question 7.
Other:
•
Budget changes as the study teams attempt to
order/secure product to reserve for trial enrollment.
•
Hesitate to open trials with drugs that have shortages.
•
Had to delay opening some trials due to availability of
standard of care drugs. Most resolved at this point.

New Information and Survey Results from June 2024
9. What policy solutions would you like to see enacted to address oncology drug shortages? Please check all that apply:
(N=28)
75%
64% 64%
39% 39%
4% 4%
7%
Economic
incentives to
encourage high
quality
manufacturing of
generics (tax
incentives for
manufacturers)
A broad buffer
stock payment like
the one previously
proposed by the
Centers for
Medicare and
Medicaid Services
(CMS)
Better information
systems to
accredit/rate
generics suppliers
so hospitals can
contract with those
using high quality
practice
Incentives for
hospitals to use
high quality
suppliers
Restrictions on
Group Purchasing
Organizations
(GPOs) from
reducing generic
pricing below a
certain floor.
A narrow buffer
stock payment
impacting only
certain small
hospitals like the
one currently
proposed by CMS
Other, please
specify
Not sure
0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
Comments:
•
For any hospital incentives, we recommend to not
require additional work from hospitals, such as
submitting data on manufacturer generics
purchased. It would be ideal if this type of
information could be extracted from claims.
•
Avoid having a single manufacturer on the market
for drugs like BCG, vinblastine, and venofer.
•
When manufacturers bring to market a high dollar
therapy consider a companion responsibility to
manufacture at least one drug regularly on the
shortage list.
•
Combat the economic principles of a perfectly
competitive market which drive prices to zero
profitability.
