
1
UNITED STATES DISTRICT COURT
FOR THE EASTERN DISTRICT OF TEXAS
SHERMAN DIVISION
UNITED STATES OF AMERICA,
ex rel. STF, LLC,
Plaintiff,
v.
CHRISTOPHER GROTTENTHALER, SUSAN
HERTZBERG, JEFFREY “BOOMER” CORNWELL,
STEPHEN KASH, MATTHEW THEILER, WILLIAM
TODD HICKMAN, COURTNEY LOVE, LAURA
HOWARD, CHRISTOPHER GONZALES, JEFFREY
MADISON, PEGGY BORGFELD, STANLEY JONES,
JEFFREY PARNELL, THOMAS GRAY HARDAWAY,
RUBEN MARIONI, JORDAN PERKINS, GINNY
JACOBS, SCOTT JACOBS, ASCEND
PROFESSIONAL MANAGEMENT, INC., ASCEND
PROFESSIONAL CONSULTING, INC., BENEFITPRO
CONSULTING LLC, NEXT LEVEL HEALTHCARE
CONSULTANTS LLC, LGRB MANAGEMENT
SERVICES LLC, S&G STAFFING, LLC, and JACOBS
MARKETING, INC.,
Defendants.
Civil Action No. 4:16-CV-547
UNITED STATES’
COMPLAINT AND
DEMAND FOR JURY TRIAL
FILED UNDER SEAL
The United States of America, for its complaint, states:
NATURE OF ACTION
1. This is an action against laboratory and hospital executives, employees, and
recruiters to recover treble damages and civil penalties under the False Claims Act (FCA), 31
U.S.C. §§ 3729–33, and to recover money for common law or equitable causes of action for
payment by mistake and unjust enrichment.
2. From at least 2010 to 2014, various laboratories encouraged healthcare providers
to order blood tests by directly paying providers kickbacks disguised as processing and handling
(P&H) fees. Laboratories competed to offer the highest P&H fees to providers, topped by Health
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 1 of 154 PageID #: 884
2
Diagnostics Laboratory, Inc. (HDL) paying $20 per referral. Various FCA suits against
laboratories and individual defendants were filed regarding these kickbacks to referring providers,
including FCA suits against HDL and Boston Heart Diagnostics Corporation (BHD). In June 2014,
the Office of the Inspector General for the Department of Health and Human Services (HHS-OIG)
issued a special fraud alert warning about kickbacks for laboratory referrals. In April 2015, the
Department of Justice (DOJ) intervened in an FCA suit alleging that HDL and three executives
had offered and paid kickbacks for laboratory referrals. HDL settled for $47 million and each of
the three executives were found liable for over $111 million in a judgment affirmed in all respects
by the Fourth Circuit, with certiorari denied by the Supreme Court. Similarly, BHD paid over $26
million to settle allegations of paying P&H fees and other kickbacks.
3. Despite HHS-OIG’s published warning and DOJ’s enforcement action, a new
laboratory kickback scheme began in or about August 2014, just two months after HHS-OIG’s
special fraud alert. The kickback scheme involved payments to healthcare providers through
purported management services organizations (MSOs) to induce the providers’ laboratory
referrals. The MSO kickback scheme began by executives Jeffrey Madison and Peggy Borgfeld at
Rockdale Hospital d/b/a Little River Healthcare (LRH), a small critical access hospital system
based in Rockdale, Texas. While the MSO kickback scheme initially concerned toxicology testing,
it expanded to include diagnostic blood testing in or about May 2015, just one month after DOJ’s
enforcement action against HDL and three executives.
4. Through the MSO kickback scheme, many of those previously involved in the
laboratory P&H fee kickback scheme continued to use kickbacks to induce laboratory referrals.
Both BHD and HDL’s successor, True Health Diagnostics, LLC (THD), joined and participated
in the MSO kickback scheme. So did their executives, including THD’s Chief Executive Officer
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 2 of 154 PageID #: 885
3
(CEO) Christopher Grottenthaler, Vice President (VP) of Sales Boomer Cornwell, Director of
Strategic Accounts Stephen Kash, and Account Executive Courtney Love, and BHD’s CEO Susan
Hertzberg, VP of Sales Matthew Theiler, and Area Sales Manager Laura Howard. Cornwell, Kash,
Love, and Howard each had worked as employees or contractors for HDL and had offered P&H
fee kickbacks to providers in Texas. With their new kickback schemes, they targeted many of the
same providers who had received P&H fee kickbacks.
5. In the MSO kickback scheme, BHD and THD conspired with small Texas hospitals
to submit false claims to Medicare, Medicaid, and TRICARE. Pursuant to the kickback scheme,
the hospitals paid recruiters to arrange for and recommend referrals, and the recruiters kicked back
a portion of the hospital payments to the referring providers who ordered BHD or THD laboratory
tests from the hospitals or from BHD or THD themselves. BHD and THD, though competitors,
worked with the same hospitals and recruiters to pay kickbacks to providers. Their executives and
sales force leveraged the MSO kickbacks to gain and increase provider referrals and, in turn, to
increase their own pay. To increase reimbursement, one of the hospitals, LRH, falsely billed the
laboratory tests as hospital outpatient services. Moreover, as part of the scheme, providers were
encouraged by the laboratories, hospitals, and recruiters to routinely order large panels of
laboratory tests for patients, even when not reasonable and necessary.
6. In addition to the MSO kickback scheme, numerous defendants participated in
additional schemes to pay kickbacks in the form of (a) P&H fees to draw site companies that were
purportedly independent of referring providers, but in fact were conduits to pay P&H fees to
providers and their employees to induce referrals for laboratory testing; (b) monthly fees to a high-
referring provider, disguising the payments as consulting fees for participating in THD’s advisory
board, even though no such board actually existed at THD; and (c) waiving patient copayments
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 3 of 154 PageID #: 886

4
and deductibles. These kickbacks were paid to induce referrals to federal healthcare programs for
laboratory testing.
7. Further, numerous defendants knowingly submitted and/or caused LRH and THD
to submit to Medicare claims for laboratory testing that were improperly referred by physicians
with a financial relationship with LRH and THD, respectively, in violation of the physician self-
referral law (commonly referred to as the Stark Law). The laboratory testing referrals were
improper because the physicians had financial relationships with LRH or THD that did not satisfy
any applicable Stark Law exception.
8. Lastly, defendants arranged for and recommended that healthcare providers
routinely order laboratory testing from THD, BHD, and LRH without regard to specific patient
needs, and encouraged providers to order laboratory tests that were not reasonable and necessary
for the diagnosis or treatment of any illness or injury of the patient or to improve the functioning
of any malformed body member of the patient.
JURISDICTION AND VENUE
9. This action arises under the FCA and the common law.
10. This Court has subject matter jurisdiction over this action under 28 U.S.C. § 1345
because the United States is the plaintiff. The Court also has subject matter jurisdiction over this
action under 28 U.S.C. §§ 1331 and 1367(a).
11. The Court may exercise personal jurisdiction over the defendants under 31 U.S.C.
§ 3732(a) because acts proscribed by the FCA, 31 U.S.C. § 3729, occurred in this District, and one
or more defendants can be found, reside, or transact business in this District.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 4 of 154 PageID #: 887

5
12. Venue is proper in the Eastern District of Texas under 31 U.S.C. § 3732(a) and 28
U.S.C. § 1391(b) because a substantial part of the events giving rise to this action occurred in this
District, and one or more defendants can be found, reside, or transact business in this District.
PARTIES
13. Plaintiff, the United States of America, acting through the Department of Health
and Human Services (HHS), administers the Health Insurance Program for the Aged and Disabled
established by Title XVIII of the Social Security Act (SSA), 42 U.S.C. §§ 1395 et seq. (Medicare),
and Grants to States for Medical Assistance Programs pursuant to Title XIX of the Act, 42 U.S.C.
§§ 1396 et seq. (Medicaid). The United States, acting through the Defense Health Agency (DHA),
administers the TRICARE program (formerly CHAMPUS). Relator STF, LLC has filed this case
under the FCA’s qui tam provisions, and the United States has intervened in part, declined in part,
and added additional claims pursuant to 31 U.S.C. § 3731(c).
14. Relator STF, LLC is a limited liability company, whose members are Felice Gersh,
M.D. and Chris Riedel.
15. Defendant Christopher Grottenthaler was the founder and CEO of THD, Outreach
Management Solutions LLC d/b/a True Health Outreach (THD-Outreach), and Health Core
Financial LLC d/b/a True Health Financial (THD-Financial). During the relevant period, he
resided in Frisco, Texas, and the headquarters for THD, THD-Outreach, and THD-Financial were
in Frisco, Texas.
16. Defendant Susan Hertzberg was BHD’s Chief Executive Officer. She oversaw
BHD’s business in Texas, including its relationship with LRH. Hertzberg transacts business in
Texas and is CEO and director of BrainScope Company, Inc., a company registered to do business
in Texas.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 5 of 154 PageID #: 888
6
17. Defendant Jeffrey “Boomer” Cornwell resides in McKinney, Texas, in this District,
and was hired by and reported to Grottenthaler as THD’s VP of Sales for the Southwestern Region,
which included the State of Texas.
18. Defendant Stephen Kash resides in Beaumont, Texas and was hired by and reported
to Grottenthaler as THD’s Director of Strategic Accounts. Kash also was a recruiter for MSOs that
paid kickbacks to providers in Texas, including in this District.
19. Defendant Matthew Theiler was BHD’s VP of Sales. In that role, he supervised
BHD employees responsible for sales in Texas, including in this District.
20. Defendant William Todd Hickman resides in Lumberton, Texas and owned and
operated defendants Ascend Professional Management, Inc. (APM) and Ascend Professional
Consulting, Inc. (APC), each of which was a corporation incorporated in Texas with its principal
place of business in Texas. Hickman also owned and operated defendant BenefitPro Consulting
LLC (BenefitPro), a company formed in Texas with its principal place of business in Texas.
21. Defendant Courtney Love resides in Dallas, Texas. She was a THD Account
Executive in Texas, and her sales territory included this District.
22. Defendant Laura Howard resides in Allen, Texas. She was a BHD Area Sales
Manager, whose sales territory included this District. She also was a recruiter for MSOs that paid
kickbacks to providers in Texas, including in this District.
23. Defendant Christopher Gonzales resides in McKinney, Texas. He was a recruiter
for MSOs that paid kickbacks to providers in Texas, including in this District.
24. Defendant Jeffrey Madison resides in Georgetown, Texas and was the CEO of
LRH, which was headquartered in Rockdale, Texas.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 6 of 154 PageID #: 889

7
25. Defendant Peggy Borgfeld resides in Lexington, Texas and at various points during
the relevant period was LRH’s Chief Financial Officer (CFO) and Chief Operations Officer
(COO).
26. Defendant Stanley Jones resides in San Antonio, Texas, defendant Jeffrey Parnell
resides in Dallas, Texas, and defendant Thomas Gray Hardaway resides in San Antonio, Texas.
Jones, Parnell, and Hardaway owned and operated defendant LGRB Management Services LLC
(LGRB), which was formed in Texas with its principal place of business in Texas.
27. Defendant Ruben Marioni resides in Spring, Texas, and defendant Jordan Perkins
resides in Conroe, Texas. Marioni and Perkins owned and operated defendant Next Level
Healthcare Consultants LLC (Next Level), which was formed in Texas with its principal place of
business in Texas.
28. Defendants Ginny Jacobs and Scott Jacobs reside in Magnolia, Texas. They owned
and operated defendant S&G Staffing, LLC (S&G), a company formed in Texas with its principal
place of business in Texas, and defendant Jacobs Marketing, Inc. (Jacobs Marketing), a corporation
incorporated in Texas with its principal place of business in Texas.
LEGAL AND REGULATORY BACKGROUND
I. THE FALSE CLAIMS ACT
29. The FCA provides, in pertinent part, that any person who:
(a)(1)(A) knowingly presents, or causes to be presented, a false or fraudulent
claim for payment or approval;
(a)(1)(B) knowingly makes, uses, or causes to be made or used, a false record
or statement material to a false or fraudulent claim; [or]
(a)(1)(C) conspires to commit a violation of subparagraph (A) [or] (B) . . .
is liable to the United States for three times the amount of damages which the United States
sustains, plus a civil penalty per violation. 31 U.S.C. § 3729(a)(1).
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 7 of 154 PageID #: 890
8
30. FCA penalties are regularly adjusted for inflation, pursuant to the Federal Civil
Penalties Inflation Adjustment Act Improvements Act of 2015. See 28 U.S.C. § 2461 note. For
violations occurring between September 28, 1999 and November 2, 2015, the civil penalty
amounts range from a minimum of $5,500 to a maximum of $11,000. See 28 C.F.R. § 85.3; 64
Fed. Reg. 47099, 47103 (1999). For violations occurring after November 2, 2015, the civil penalty
amounts currently range from a minimum of $11,803 to a maximum of $23,607. 28 C.F.R. § 85.5.
31. For purposes of the FCA, the terms “knowing” and “knowingly”
(A) mean that a person, with respect to information—
(i) has actual knowledge of the information;
(ii) acts in deliberate ignorance of the truth or falsity of the information; or
(iii) acts in reckless disregard of the truth or falsity of the information; and
(B) require no proof of specific intent to defraud.
31 U.S.C. § 3729(b)(1).
32. Under the FCA, a “claim” includes direct requests to the United States for payment
as well as reimbursement requests made to the recipients of federal funds under federal benefits
programs. 31 U.S.C. § 3729(b)(2)(A).
33. The FCA defines “material” to mean “having a natural tendency to influence, or be
capable of influencing, the payment or receipt of money or property.” 31 U.S.C. § 3729(b)(4).
II. THE MEDICARE PROGRAM
34. In 1965, Congress enacted the Health Insurance for the Aged and Disabled Act,
known as the Medicare program, to pay for the costs of certain healthcare services. 42 U.S.C.
§ 1395 et seq. Entitlement to Medicare benefits is based on age, disability, or affliction with end-
stage renal disease. See 42 U.S.C. §§ 426 to 426-1.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 8 of 154 PageID #: 891
9
35. HHS is responsible for administration and supervision of the Medicare program.
The Centers for Medicare & Medicaid Services (CMS), an agency within HHS, is directly
responsible for administering the Medicare program.
36. To participate in the Medicare program, a healthcare provider must file an
agreement with the Secretary of HHS. 42 U.S.C. § 1395cc. The agreement requires compliance
with the requirements that the Secretary deems necessary for participation in the Medicare program
in order to receive reimbursement from Medicare.
37. To enroll in the Medicare program, suppliers of laboratory services must submit a
Medicare Enrollment Application, Form CMS-855B. These providers also must complete Form
CMS-855B to change information or to reactivate, revalidate, and/or terminate Medicare
enrollment.
38. Form CMS-855B requires, among other things, signatories to certify:
I agree to abide by the Medicare laws, regulations and program
instructions that apply to me or to the organization listed in section
2A1 of this application. The Medicare laws, regulations, and
program instructions are available through the Medicare
Administrative Contractor. I understand that payment of a claim by
Medicare is conditioned upon the claim and the underlying
transaction complying with such laws, regulations and program
instructions . . . .
* * *
I will not knowingly present or cause to be presented a false or
fraudulent claim for payment by Medicare, and I will not submit
claims with deliberate ignorance or reckless disregard of their truth
or falsity.
See https://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/Downloads/cms855b.pdf.
39. An authorized official must sign the “Certification Statement” in Section 15 of
Form CMS-855B, which “legally and financially binds this supplier to the laws, regulations, and
program instructions of the Medicare program.” Id.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 9 of 154 PageID #: 892
10
40. To enroll in the Medicare program, institutional providers such as hospitals must
submit a Medicare Enrollment Application, Form CMS-855A. These providers also must complete
Form CMS-855A to change information or to reactivate, revalidate, and/or terminate Medicare
enrollment.
41. Form CMS 855A requires, among other things, signatories to certify:
I agree to abide by the Medicare laws, regulations and program
instructions that apply to this provider. . . . I understand that payment
of a claim by Medicare is conditioned upon the claim and the
underlying transaction complying with such laws, regulations, and
program instructions (including, but not limited to, the Federal anti-
kickback statute and the Stark law), and on the provider’s
compliance with all applicable conditions of participation in
Medicare.
* * *
I will not knowingly present or cause to be presented a false or
fraudulent claim for payment by Medicare, and I will not submit
claims with deliberate ignorance or reckless disregard of their truth
or falsity.
See https://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/Downloads/cms855a.pdf.
42. An authorized official must sign the “Certification Section” in Section 15 of Form
CMS-855A, which “legally and financially binds [the] provider to the laws, regulations, and
program instructions of the Medicare program.” Id.
43. In addition, within five months of the end of the cost reporting period, hospitals are
required to submit to CMS annual reports known as “cost reports” on Form CMS-2552, see 42
C.F.R. §§ 413.20(b), 413.24(f)(2). The top of Form CMS-2552 states:
This report is required by law (42 USC 1395g; 42 CFR 413.20(b)).
Failure to report can result in all interim payments made since the
beginning of the cost reporting period being deemed overpayments
(42 USC 1395g).
https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/R3P240f.pdf.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 10 of 154 PageID #: 893
11
44. Part II of Form CMS-2552 and 42 C.F.R. § 413.24(f)(4)(iv)(B) require a mandatory
certification, which includes the following certification statement:
MISREPRESENTATION OR FALSIFICATION OF ANY
INFORMATION CONTAINED IN THIS COST REPORT MAY
BE PUNISHABLE BY CRIMINAL, CIVIL AND
ADMINISTRATIVE ACTION, FINE AND/OR IMPRISONMENT
UNDER FEDERAL LAW. FURTHERMORE, IF SERVICES
IDENTIFIED IN THIS REPORT WERE PROVIDED OR
PROCURED THROUGH THE PAYMENT DIRECTLY OR
INDIRECTLY OF A KICKBACK OR WERE OTHERWISE
ILLEGAL, CRIMINAL, CIVIL AND ADMINISTRATIVE
ACTION, FINES AND OR IMPRISONMENT MAY RESULT.
Id.
45. Form CMS-2552 and 42 C.F.R. § 413.24(f)(4)(iv)(B) require a chief financial
officer or administrator of the hospital to certify that “I have read the above certification statement
and that I have examined the accompanying electronically filed or manually submitted cost report
and the Balance Sheet and Statement of Revenue and Expenses prepared by [Provider Name(s)
and Number(s)] for the cost reporting period beginning [date] and ending [date] and to the best of
my knowledge and belief, this report and statement are true, correct, complete and prepared from
the books and records of the provider in accordance with applicable instructions, except as noted.”
Id.
46. Form CMS-2552 and 42 C.F.R. § 413.24(f)(4)(iv)(B) also require a chief financial
officer or administrator of the hospital to certify that “I am familiar with the laws and regulations
regarding the provision of health care services, and that the services identified in this cost report
were provided in compliance with such laws and regulations.” Id.
47. To enroll in the Medicare program, physicians must submit a Medicare Enrollment
Application, Form CMS-855I. These providers also must complete Form CMS-855I to change
information or to reactivate, revalidate, and/or terminate Medicare enrollment.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 11 of 154 PageID #: 894
12
48. Form CMS-855I requires, among other things, signatories to certify:
I agree to abide by the Medicare laws, regulations and program
instructions that apply to me or to the organization listed in section
4A of this application. The Medicare laws, regulations, and program
instructions are available through the Medicare Administrative
Contractor. I understand that payment of a claim by Medicare is
conditioned upon the claim and the underlying transaction
complying with such laws, regulations and program instructions
(including, but not limited to, the Federal Anti-Kickback Statute, 42
U.S.C. section 1320a-7b(b) (section 1128B(b) of the Social Security
Act) and the Physician Self-Referral Law (Stark Law), 42 U.S.C.
section 1395nn (section 1877 of the Social Security Act)).
* * *
I will not knowingly present or cause to be presented a false or
fraudulent claim for payment by Medicare and will not submit
claims with deliberate ignorance or reckless disregard of their truth
or falsity.
See https://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/Downloads/cms855i.pdf.
49. The provider must sign the “Certification Section” in Section 15 of Form CMS-
855I, and in doing so, is “attesting to meeting and maintaining the Medicare requirements”
excerpted above, among others. Id.
50. Medicare reimburses only those services furnished to beneficiaries that are
“reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the
functioning of a malformed body member . . . .” 42 U.S.C. § 1395y(a)(l)(A).
51. The Secretary of HHS (Secretary) is responsible for specifying services covered
under the “reasonable and necessary” standard and has wide discretion in selecting the means for
doing so. See 42 U.S.C. § 1395ff(a). The Secretary acts through formal regulations, and
periodically CMS and HHS-OIG issue industry guidance.
52. The Secretary provides guidance to eligible providers pursuant to a series of
Manuals, published by CMS, which are available to the public on the Internet. See generally CMS
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 12 of 154 PageID #: 895
13
Internet-Only Manuals, available at https://www.cms.gov/regulations-and-guidance/guidance/
manuals/internet-only-manuals-ioms.html.
53. At all times relevant to this Complaint, CMS contracted with private contractors,
known as Medicare Administrative Contractors (MACs), to perform various administrative
functions on its behalf, including reviewing and paying claims submitted by healthcare providers.
42 U.S.C. §§ 1395h, 1395u; 42 C.F.R. §§ 421.3, 421.100, 421.104. MACs generally act on behalf
of CMS to process and pay Medicare claims and perform administrative functions on a regional
level. MACs may issue Local Coverage Determinations regarding whether or not a particular item
or service is covered. 42 U.S.C. § 1395ff(f)(2).
54. Medicare regulations require providers and suppliers to certify that they meet, and
will continue to meet, the requirements of the Medicare statute and regulations. 42 C.F.R.
§ 424.516(a)(1). In submitting claims for payment to Medicare, providers must certify that the
information on the claim form accurately describes the services rendered and that the services were
reasonable and medically necessary for the patient.
55. To obtain Medicare reimbursement, healthcare providers (including suppliers)
submit claims using paper forms or their electronic equivalents. Providers identify by code on the
appropriate form, among other things, the principal diagnosis of the patient and the procedures and
services rendered.
A. Medicare Part A
56. Under Medicare Part A, hospitals agree with Medicare to provide covered
healthcare items and services to treat Medicare patients. The hospital, also called a “provider,” is
authorized to bill Medicare for that treatment. During the relevant time period, CMS reimbursed
hospitals for inpatient Part A services through MACs (formerly known as fiscal intermediaries).
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 13 of 154 PageID #: 896
14
57. Since 2007, in order to get paid, a hospital must complete and submit to the MAC
a claim for payment on a Form UB-04 (also known as CMS-1450) or its electronic equivalent.
This form contains patient-specific information including the diagnosis and types of services that
are assigned or provided to the Medicare patient. The Medicare program relies on the accuracy
and truthfulness of the UB-04 Forms to determine whether the service is payable and the amounts,
if any, the hospital is owed or has been overpaid.
58. In addition, as noted previously, hospitals are required to submit to the MAC an
annual report known as a Medicare “cost report” on Form CMS-2552, which identifies any
outstanding costs that the hospital is claiming for reimbursement for that year. The cost report
serves as the final claim for payment that is submitted to Medicare. Failure to submit a cost report
can result in all interim payments made since the beginning of the cost reporting period being
deemed overpayments. The Medicare program relies on the accuracy and truthfulness of the cost
report to determine the amounts, if any, the hospital is owed or has been overpaid during the year.
B. Medicare Part B
59. Part B of the Medicare program is a federally subsidized, voluntary insurance
program that pays for various medical and other health services and supplies, including laboratory
testing, hospital outpatient services, physician services, and physical, occupational, and speech
therapy services. See 42 U.S.C. §§ 1395j to 1395w-5.
60. Medicare Part B is funded by insurance premiums paid by enrolled Medicare
beneficiaries and by contributions from the Federal Treasury. Eligible individuals who are 65 or
older or disabled may enroll in Medicare Part B to obtain benefits in return for payments of
monthly premiums. Payments under Medicare Part B typically are made directly under assignment
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 14 of 154 PageID #: 897
15
to service providers and practitioners, such as physicians, rather than to the patient/beneficiary. In
that case, the physician bills the Medicare program directly.
61. CMS provides reimbursement for Medicare Part B claims from the Medicare Trust
Fund. To assist in the administration of Medicare Part B, CMS contracts with MACs (formerly
known as carriers). 42 U.S.C. § 1395u. MACs perform various administrative functions for CMS,
including processing the payment of Medicare Part B claims to providers.
62. To obtain Medicare reimbursement for certain outpatient items or services,
providers and suppliers submit a claim form known as the CMS 1500 form or its electronic
equivalent, known as the 837P format. When a CMS-1500 claim is submitted, the provider certifies
that he or she is knowledgeable of Medicare’s requirements and that the services for which
payment is sought were “medically indicated and necessary for the health of the patient.”
63. Providers wishing to submit an electronic or hard-copy CMS-1500 claim must first
seek to enroll in the Medicare program by submitting a provider enrollment form. During the
Medicare enrollment process, providers must certify that the claims they submit will be “accurate,
complete, and truthful.”
64. For a claim to be paid by Medicare Part B, it must identify each service rendered to
the patient by the provider. The service is identified by a code in an American Medical Association
(AMA) publication called the Current Procedural Terminology (CPT) Manual. The CPT Manual
is a systematic list of codes for procedures and services performed by or at the direction of a
provider. Each procedure or service is identified by a five-digit CPT code.
65. In addition to the CPT Manual, the AMA publishes the International Classification
of Diseases (ICD) Manual, which assigns a unique numeric identifier to each medical condition.
To be payable by Medicare, the claim must identify both the CPT code that the provider is billing
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 15 of 154 PageID #: 898
16
for and the corresponding ICD code(s) for the patient’s medical condition that supports the medical
necessity of the provider’s service.
66. When submitting claims on the CMS-1500 to Medicare, providers certify, among
other things, that: (a) the services rendered are medically indicated and necessary for the health
of the patient; (b) the information in the claim is “true, accurate, and complete”; and (c) the
provider understands that “payment and satisfaction of this claim will be from Federal and State
funds, and that any false claims, statements, or documents, or concealment of material fact, may
be prosecuted under applicable Federal and State laws.” After a February 2012 revision to the
CMS-1500, providers further certify that their claims comply “with all applicable Medicare and/or
Medicaid laws, regulations, and program instructions for payment including but not limited to the
Federal anti-kickback statute and Physician Self-Referral law (commonly known as Stark law).”
CMS-1500 also requires providers to acknowledge that: “Any person who knowingly files a
statement of claim containing any misrepresentation or any false, incomplete or misleading
information may be guilty of a criminal act punishable under law and may be subject to civil
penalties.”
67. When enrolling to submit claims electronically, providers certify that they will
submit claims that are “accurate, complete, and truthful.” When a provider submits an electronic
claim, the provider’s identification number and password serve as the provider’s signature, just as
if the provider physically signed the claim form.
68. Healthcare providers are prohibited from knowingly presenting or causing to be
presented claims for items or services that the person knew or should have known were not
medically necessary, or knew or should have known were false or fraudulent. E.g., 42 U.S.C.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 16 of 154 PageID #: 899

17
§ 1395y(a)(1)(A); 42 U.S.C. § 1320a-7(b)(7) (providers may be excluded for fraud, kickbacks, and
other prohibited activities).
69. A provider has a duty to familiarize itself with the statutes, regulations, and
guidelines regarding coverage for the Medicare services it provides. Heckler v. Cmty. Health Servs.
of Crawford Cty., Inc., 467 U.S. 51, 64 (1984).
70. Because it is not feasible for the Medicare program or its contractors to review
medical records corresponding to each of the millions of claims for payment it receives from
providers, the program relies on providers to comply with Medicare requirements and relies on
providers to submit truthful and accurate certifications and claims.
71. Generally, once a provider submits a CMS-1500 or the electronic equivalent to the
Medicare program, the claim is paid directly to the provider, in reliance on the foregoing
certifications, without any review of supporting documentation, including medical records.
III. TEXAS MEDICAID PROGRAM
72. State Medicaid programs are authorized by the Social Security Act, Title XIX. 42
U.S.C. §§ 1396 et seq. Medicaid is a joint federal-state program that provides healthcare benefits
for certain groups including the poor and disabled. Each state Medicaid program must implement
a “State Plan” containing specified minimum criteria for coverage and payment of claims to qualify
for federal funds for Medicaid expenditures. 42 U.S.C. § 1396a.
73. The federal portion of each state’s Medicaid payments, known as the Federal
Medical Assistance Percentage (FMAP), is based on a state’s per capita income compared to the
national average. 42 U.S.C. § 1396d(b). During the relevant time period, the federal portion of
Medicaid payments for Texas is set forth below:
Time Period
Texas FMAP
10/1/14 - 9/30/15
58.05%
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 17 of 154 PageID #: 900

18
Time Period Texas FMAP
10/1/15 - 9/30/16 57.13%
10/1/16 - 9/30/17
56.18%
10/1/17 - 9/30/18 56.88%
10/1/18 - 9/30/19 58.19%
79 Fed. Reg. 3385, 3387 (Jan. 21, 2014) (FY 2015); 79 Fed. Reg. 71,426, 71,428 (Dec. 2, 2014)
(FY 2016); 80 Fed. Reg. 73,779, 73,781–82 (Nov. 25, 2015) (FY 2017); 81 Fed. Reg. 80,078,
80,080 (Nov. 15, 2016) (FY 2018); 82 Fed. Reg. 55,383, 55,385 (Nov. 21, 2017) (FY 2019).
74. The Texas Health and Human Services Commission (HHSC) is responsible for
administering the Medicaid program in the State of Texas. HHSC contracts with the Texas
Medicaid and Healthcare Partnership (TMHP) to receive applications from prospective Medicaid
providers, assign Medicaid provider numbers, educate providers as to Medicaid policies and
regulations, and process and pay Medicaid claims. TMHP has issued Texas Medicaid Provider
Manuals for the purpose of furnishing Medicaid providers with the policies and procedures needed
to receive reimbursement for covered services provided to eligible Texas Medicaid recipients.
Throughout the relevant time period, the Texas Medicaid Provider Manuals were available for
review at the State office and in each local and district office, as well as online at
https://www.tmhp.com/resources/provider-manuals/tmppm.
75. To participate in the Texas Medicaid program, providers such as physicians and
hospitals must certify in their Medicaid provider agreement that they will “agree[] to abide by all
Medicaid regulations, program instructions, and Title XIX of the Social Security Act” and “comply
with all of the requirements of the [Texas Medicaid] Provider Manual, as well as all state and
federal laws governing or regulating Medicaid.”
76. Providers participating in the Texas Medicaid program must certify that they
“understand[] that payment of a claim by Medicaid is conditioned upon the claim and the
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 18 of 154 PageID #: 901
19
underlying transaction complying with such laws, regulations, and program instructions
(including, but not limited to, the Federal anti-kickback statute and the Stark law), and on the
provider’s compliance with all applicable conditions of participation in Medicaid.”
77. To receive payments from the Texas Medicaid program, providers must agree that
“information submitted regarding claims or encounter data will be true, accurate, and complete,
and that the Provider’s records and documents are both accessible and validate the services and
the need for services billed and represented as provided.” Likewise, such providers must
acknowledge that they have “an affirmative duty to verify that claims and encounters submitted
for payment are true and correct” and that “payments received are for actual services rendered and
medically necessary.”
78. Pursuant to Texas regulations, the Texas Medicaid program covers medical
services, including laboratory testing, only if the services are “medically necessary for diagnosis
or treatment, or both, of illness or injury” or “appropriately authorized for prevention of the
occurrence of a medical condition, and is prescribed by a physician or other qualified practitioner,
as appropriate to the particular benefit, in accordance with federal or state law or policy and the
[Texas Medicaid] utilization review provisions of this chapter.” 1 Texas Admin. C. § 354.1131(a).
79. A laboratory enrolled as a Texas Medicaid provider must submit claims on a CMS-
1500 claim form or its electronic equivalent, which contains the certifications in Section II above.
80. A hospital enrolled as a Texas Medicaid provider must submit claims on a UB-04
claim form, CMS-2552 form, or its electronic equivalent, which contain the certifications in
Section II above.
81. Because it is not feasible for the Texas Medicaid program or its contractors to
review medical records corresponding to each of the claims for payment it receives from providers,
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 19 of 154 PageID #: 902
20
the program relies on providers to comply with Medicaid requirements and relies on providers to
submit truthful and accurate certifications and claims.
IV. THE TRICARE PROGRAM
82. DHA administers TRICARE (formerly CHAMPUS), a medical benefits program
established by federal law. 10 U.S.C. §§ 1071–1110b. TRICARE covers eligible beneficiaries,
including active duty members of the Uniformed Services and their dependents as well as retired
members of the Uniformed Services and their dependents. The federal government reimburses a
portion of the cost of covered healthcare services and prescription medications provided to
TRICARE beneficiaries.
83. TRICARE covers only medically necessary care; specifically, services or supplies
provided by a hospital, physician, and/or other provider for the prevention, diagnosis, and
treatment of an illness, when those services or supplies are determined to be consistent with the
condition, illness, or injury; are provided in accordance with approved and generally accepted
medical or surgical practice; are not primarily for the convenience of the patient, the physician, or
other providers; and do not exceed (in duration or intensity) the level of care which is needed to
provide safe, adequate, and appropriate diagnosis and treatments. See 32 C.F.R. § 199.4(a)(1)(i)
and applicable definitions at 32 C.F.R. § 199.2.
84. Federal regulations provide that TRICARE may deny payment in “abuse
situations.” 32 C.F.R. § 199.9(b). To avoid abuse situations, providers are obligated to provide
services and supplies under TRICARE that are: “Furnished at the appropriate level and only when
and to the extent medically necessary . . .; of a quality that meets professionally recognized
standards of health care; and, supported by adequate medical documentation as may reasonably be
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 20 of 154 PageID #: 903
21
required under this part . . . to evidence the medical necessity and quality of services furnished, as
well as the appropriateness of the level of care.” Id.
85. TRICARE regulations, in turn, define “appropriate” medical care as that which is,
among other things, “[f]urnished economically”—i.e., “in the least expensive level of care or
medical environment adequate to provide the required medical care.” 32 C.F.R. § 199.2.
86. As with Medicare, providers submit claims to TRICARE using the CMS-1500 or
an electronic equivalent. Providers therefore make the same certifications in submitting claims to
TRICARE as they do when submitting claims to Medicare.
87. Because it is not feasible for the TRICARE program or its contractors to review
medical records corresponding to each of the claims for payment it receives from providers, the
program relies on providers to comply with TRICARE requirements and submit truthful and
accurate certifications and claims.
V. THE ANTI-KICKBACK STATUTE
88. The Anti-Kickback Statute (AKS), 42 U.S.C. § 1320a-7b(b), arose out of
Congressional concerns involving physicians’ conflicts of interest and overutilization of medical
services and items. First enacted in 1972, Congress strengthened the statute in 1977 and 1987 to
ensure that kickbacks masquerading as legitimate transactions did not evade its reach. See Social
Security Amendments of 1972, Pub. L. No. 92-603, § 242(b), (c); 42 U.S.C. § 1320a-7b, Medicare-
Medicaid Anti-Fraud and Abuse Amendments, Pub. L. No. 95-142; Medicare and Medicaid
Patient and Program Protection Act of 1987, Pub. L. No. 100-93. The AKS prohibits kickback
payments to protect the integrity of federal healthcare programs such as Medicare, Medicaid, and
TRICARE.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 21 of 154 PageID #: 904
22
89. The AKS prohibits any person from knowingly and willfully offering, paying,
soliciting, or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in
kind, to induce or reward a person for, inter alia, purchasing, ordering, arranging for, or
recommending the purchase or ordering of any goods or services for which payment may be made,
in whole or in part, under a federal healthcare program.
90. In pertinent part, the AKS provides:
b. Illegal remunerations
(1) Whoever knowingly and willfully solicits or receives any remuneration
(including any kickback, bribe, or rebate) directly or indirectly, overtly or
covertly, in cash or in kind—
(A) in return for referring an individual to a person for the furnishing or
arranging for the furnishing of any item or service for which payment may
be made in whole or in part under a Federal health care program, or
(B) in return for purchasing, leasing, ordering, or arranging for or
recommending purchasing, leasing, or ordering any good, facility, service,
or item for which payment may be made in whole or in part under a Federal
health care program,
shall be guilty of a felony and upon conviction thereof, shall be fined not
more than $100,000 or imprisoned for not more than ten years, or both.
(2) Whoever knowingly and willfully offers or pays any remuneration
(including any kickback, bribe, or rebate) directly or indirectly, overtly or
covertly, in cash or in kind to any person to induce such person
(A) to refer an individual to a person for the furnishing or arranging for the
furnishing of any item or service for which payment may be made in whole
or in part under a Federal health care program, or
(B) to purchase, lease, order or arrange for or recommend purchasing,
leasing or ordering any good, facility, service, or item for which payment
may be made in whole or in part under a Federal health care program,
shall be guilty of a felony and upon conviction thereof, shall be fined not
more than $100,000 or imprisoned for not more than ten years, or both.
42 U.S.C. § 1320a-7b(b). “[A] person need not have actual knowledge of [the AKS] or specific
intent to commit a violation of [the AKS].” Id. § 1320a-7b(h).
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 22 of 154 PageID #: 905
23
91. Pursuant to the AKS, “a claim that includes items or services resulting from a
violation of [the AKS] constitutes a false or fraudulent claim for purposes of [the FCA].” 42 U.S.C.
§ 1320a-7b(g); see also, e.g., Guilfoile v. Shields, 913 F.3d 178, 190–91 (1st Cir. 2019) (“§ 1320a-
7b(g)’s obviation of the ‘materiality’ inquiry essentially codifies the long-standing view that AKS
violations are ‘material’ in the FCA context.”).
A. AKS “Safe Harbors”
92. The HHS Office of Inspector General (OIG) has promulgated “safe harbor”
regulations that define practices that are not subject to the AKS because such practices are unlikely
to result in fraud or abuse. 42 C.F.R. § 1001.952. The safe harbors set forth specific conditions
that, if met, assure persons involved of not being sanctioned for the arrangement qualifying for the
safe harbor. However, safe harbor protection is an affirmative defense that is afforded to only those
arrangements that meet all requirements of the safe harbor.
93. Under the investment interests safe harbor, a payment to an investor that is a return
on an investment is not remuneration for purposes of the AKS only if all eight of the safe harbor’s
requirements are satisfied. See 42 C.F.R. § 1001.952(a).
94. The safe harbor for investment interests is narrowly tailored to prevent improper
economic inducements from being disguised as ordinary investments. Among other things, the
safe harbor for investment interests requires:
• The terms on which an investment interest is offered to an investor who is in a position to
. . . generate business for the entity must not be related to the previous or expected volume
of referrals . . . or the amount of business otherwise generated from that investor to the
entity;
• No more than 40 percent of the entity’s gross revenue related to the furnishing of health
care items and services in the previous fiscal year or previous 12 month period may come
from referrals or business otherwise generated from investors;
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 23 of 154 PageID #: 906
24
• No more than 40 percent of the value of the investment interests . . . may be held in the
previous fiscal year or previous 12 month period by investors who are in a position to make
or influence referrals to . . . or otherwise generate business for the entity; [and]
• The amount of payment to an investor in return for the investment interest must be directly
proportional to the amount of the capital investment (including the fair market value of any
pre-operational services rendered) of that investor.
42 C.F.R. § 1001.952(a)(2)(i), (iii), (vi), (viii).
95. The direct and indirect payments alleged herein did not satisfy the requirements of
this or any other AKS safe harbor, and at all relevant times defendants were aware that their
conduct was unlawful.
B. OIG Special Fraud Alerts and Related Guidance
96. To alert the public to “trends of health care fraud and certain practices of an
industry-wide character,” OIG issues special fraud alerts, which are published online and in the
Federal Register. 59 Fed. Reg. 65,372, 65,373 (Dec. 19, 1994). The fraud alerts “provide general
guidance to the health care industry” and assist others “in identifying health care fraud schemes.”
Id.
97. In 1989, OIG issued a Special Fraud Alert on Joint Venture Arrangements. OIG
warned that physician joint venture arrangements may violate the AKS where the arrangement was
“intended not so much to raise investment capital legitimately to start a business, but to lock up a
stream of referrals from the physician investors and to compensate them indirectly for those
referrals.” OIG, Special Fraud Alert: Joint Venture Arrangements, reprinted in 59 Fed. Reg.
65,372, 65,374 (Dec. 19, 1994).
98. In 1994, OIG issued a Special Fraud Alert on transfers of value from laboratories
to referral sources. OIG, Special Fraud Alert: Arrangements for the Provision of Clinical
Laboratory Services, reprinted in 59 Fed. Reg. 65,372, 65,377 (Dec. 19, 1994). OIG warned of
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 24 of 154 PageID #: 907
25
“inducements offered by clinical laboratories which may implicate the [AKS],” such as providing
items, services, and financial benefits. Id. OIG warned that “[w]hen one purpose of these
arrangements is to induce the referral of program-reimbursed laboratory testing, both the clinical
laboratory and the health care provider may be liable under the [AKS] and may be subject to
criminal prosecution and exclusion from participation in the Medicare and Medicaid programs.”
Id. at 65,377–78.
99. OIG reiterated its concerns in a special bulletin in 2003 about the “proliferation of
arrangements between those in a position to refer business, such as physicians, and those providing
items or services for which Medicare or Medicaid pays.” OIG, Special Advisory Bulletin:
Contractual Joint Ventures, reprinted in 68 Fed. Reg. 23,148, 23,148 (Apr. 30, 2003) (warning
that such “questionable contractual arrangements” may violate the AKS).
100. In March 2013, OIG issued another Special Fraud Alert about physician-owned
entities, including entities “referred to as physician-owned distributorships, or ‘PODs.’” OIG
Special Fraud Alert: Physician-Owned Entities (Mar. 26, 2013), reprinted in 78 Fed. Reg. 19,271,
19,272 (Mar. 29, 2013). OIG noted that it had previously warned that physician-owned entities
create “the strong potential for improper inducements” to physician-investors and “should be
closely scrutinized under the fraud and abuse laws,” including the AKS. Id. at 19,272 (quoting
Letter from Vicki Robinson, “Response to Request for Guidance Regarding Certain Physician
Investments in the Medical Device Industries” (Oct. 6, 2006)).
101. The 2013 fraud alert reiterated longstanding AKS concerns regarding physician-
owned entities, including: (1) the corruption of medical judgment, (2) overutilization,
(3) increased costs to federal healthcare programs, and (4) unfair competition. Id. at 19,272.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 25 of 154 PageID #: 908
26
102. The 2013 fraud alert warned that PODs are “inherently suspect” under the AKS,
and it reiterated OIG’s prior guidance that providing a referring physician the opportunity to earn
a profit, including through an investment return from an entity for which the physician generates
business, could constitute illegal remuneration under the AKS. Id.
103. OIG identified the following five features, among others, that may render PODs
particularly suspect under the AKS: (1) the POD “exclusively serves its physician-owners’ patient
base,” rather than selling “on the basis of referrals from nonowner physicians”; (2) the POD
“generate[s] disproportionately high rates of return for physician-owners”; (3) the POD “enable[s]
the physician-owners to profit from their ability to dictate the [items] to be purchased for their
patients”; (4) the physician-owner(s) “are few in number, such that the volume or value of a
particular physician-owner’s recommendations or referrals closely correlates to that physician-
owner’s return on investment”; and (5) the physician-owner(s) “alter their medical practice after
or shortly before investing in the POD.” Id. at 19,273.
104. In June 2014, OIG issued a Special Fraud Alert regarding laboratory payments to
referring physicians. OIG Special Fraud Alert: Laboratory Payments to Referring Physicians (June
25, 2014), reprinted in 79 Fed. Reg. 40,115 (July 11, 2014). OIG noted that “[a]rrangements
between referring physicians and laboratories historically have been subject to abuse and were the
topic of one of the OIG’s earliest Special Fraud Alerts.” Id. at 40,116 (citing 1994 Special Fraud
Alert).
105. As OIG recognized, “the choice of laboratory, as well as the decision to order
laboratory tests, typically is made or strongly influenced by the physician, with little or no input
from patients.” Id. at 40,116. Transfers of value to physicians “may induce physicians to order
tests from a laboratory that provides them with remuneration, rather than the laboratory that
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 26 of 154 PageID #: 909
27
provides the best, most clinically appropriate service.” Id. Such transfers “also may induce
physicians to order more laboratory tests than are medically necessary, particularly when the
transfers of value are tied to, or take into account, the volume or value of business generated by
the physician.” Id.
106. With respect to P&H fees paid to physicians and physician practices in connection
with orders for laboratory tests, OIG warned that such payment arrangements “are suspect under
the [AKS].” Id. at 40,116. OIG noted that the AKS prohibits the knowing and willful payment of
remuneration “if even one purpose of the payment is to induce or reward referrals of Federal health
care program business.” Id. at 40,117. Payments to physicians are particularly suspect, OIG
indicated, when the physician is paid for services the laboratory does not actually need or for which
the physician is otherwise compensated, or when the payment is for more than fair market value
for the physician’s services or takes into account the volume or value of business generated by the
referring physician. Id. at 40,116–17.
107. Further, OIG warned of payment arrangements with physicians that purport to
“carve out” federal healthcare program beneficiaries or business. Id. at 40,117. Specifically, OIG
stated that its concerns with such payment arrangements “are not abated when those arrangements
apply only to specimens collected from non-Federal health care program patients.” Id. Rather,
“[a]rrangements that ‘carve out’ Federal health care program beneficiaries or business from
otherwise questionable arrangements implicate the anti-kickback statute and may violate it by
disguising remuneration for Federal health care program business through the payment of amounts
purportedly related to non-Federal health care program business.” Id. OIG noted that “physicians
typically wish to minimize the number of laboratories to which they refer for reasons of
convenience and administrative efficiency,” so payment arrangements “that carve out Federal
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 27 of 154 PageID #: 910
28
health care program business may nevertheless be intended to influence physicians’ referrals of
Federal health care program business to the offering laboratories.” Id.
108. OIG also warned that physicians who receive payments in connection with their
laboratory test orders “may be at risk under the [AKS]” because liability attaches to “parties on
both sides of an impermissible ‘kickback’ arrangement.” Id. at 40,117.
109. Each defendant was on notice of the foregoing Special Fraud Alerts and Bulletins
published in the Federal Register. Moreover, each defendant knew that paying kickbacks to
physicians to induce referrals of federal healthcare program business was illegal.
110. In or about June 2014, defendants Grottenthaler, Cornwell, Kash, Love, Hertzberg,
Theiler, and Howard had actual knowledge of HHS-OIG’s June 2014 Special Fraud Alert.
VI. THE STARK LAW
111. The Stark Law prohibits an entity from submitting claims to Medicare for certain
categories of “designated health services” (DHS), including clinical laboratory services, if such
services were referred to the entity by a physician with whom the entity had a financial relationship
that did not satisfy the requirements of an applicable statutory or regulatory exception. 42 U.S.C.
§ 1395nn(a)(1). The Stark Law further prohibits Medicare from paying any claims for DHS
referred in violation of the law. 42 U.S.C. § 1395nn(g)(1). The statute was designed specifically
to prevent losses that might be suffered by the Medicare program due to overutilization of DHS,
patient steering, and the corruption of physicians’ medical judgment by improper financial
incentives.
112. As initially enacted in 1989, the Stark Law applied to referrals of Medicare patients
for clinical laboratory services by a physician to a laboratory with which the physician had a
financial relationship unless the requirements of an applicable statutory or regulatory exception
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 28 of 154 PageID #: 911

29
were satisfied. See Omnibus Budget Reconciliation Act of 1989, Pub. L. No. 101-239, § 6204, 103
Stat. 2106, 2236-43. In 1993, Congress extended the Stark Law’s application to referrals for ten
additional DHS. See Omnibus Reconciliation Act of 1993, Pub. L. No. 103-66, § 13562, 107 Stat.
312, 596-605; Social Security Act Amendments of 1994, Pub. L. No. 103-432, § 152, 108 Stat.
4398, 4436-37.
113. Compliance with the Stark Law is a condition of payment by the Medicare program.
Medicare is prohibited from paying for any DHS provided in violation of the Stark Law. See 42
U.S.C. §§ 1395nn(a)(1), (g)(1). Moreover, “[a]n entity that collects payment for a designated
health service that was performed pursuant to a prohibited referral must refund all collected
amounts on a timely basis[.]” 42 U.S.C. § 411.353(d).
114. In pertinent part, the Stark Law provides:
(a) Prohibition of certain referrals
(1) In general
Except as provided in subsection (b), if a physician (or an immediate family member of
such physician) has a financial relationship with an entity specified in paragraph (2), then
(A) the physician may not make a referral to the entity for the furnishing of designated
health services for which payment otherwise may be made under this subchapter, and
(B) the entity may not present or cause to be presented a claim under this subchapter
or bill to any individual, third party payor, or other entity for designated health services
furnished pursuant to a referral prohibited under subparagraph (A).
42 U.S.C. § 1395nn(a)(1).
115. As noted above, DHS includes clinical laboratory services. 42 U.S.C.
§ 1395nn(h)(6) and 42 C.F.R. § 411.351 (2014).
1
1
The physician self-referral law regulations were amended effective on or after January 19,
2021, 85 Fed. Reg. 77,492 (Dec. 2, 2020), and on January 1, 2022, 86 Fed. Reg. 64,996 (Nov.
19, 2021). Those amendments did not apply during the relevant period in this case.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 29 of 154 PageID #: 912
30
116. Under the Stark Law, an “entity is considered to be furnishing DHS if it . . . [i]s the
person or entity that has performed services that are billed as DHS or . . . that has presented a claim
to Medicare for the DHS, including the person or entity to which the right to payment for the DHS
has been reassigned. . . .” 42 C.F.R. § 411.351 (2014).
117. A “financial relationship” includes a “compensation arrangement,” which means
any arrangement involving any “remuneration” paid to a referring physician “directly or indirectly,
overtly or covertly, in cash or in kind” by the entity furnishing the DHS. 42 U.S.C.
§§ 1395nn(h)(1)(A), (h)(1)(B); 42 C.F.R. § 411.351 (2014).
118. A direct compensation arrangement exists “if remuneration passes between the
referring physician . . . and the entity furnishing DHS without any intervening persons or entities.”
42 C.F.R. § 411.354(c)(1)(i) (2014).
119. An indirect compensation arrangement exists if (i) there is an unbroken chain of
persons or entities that have financial relationships between the referring physician and the entity
furnishing DHS; (ii) the referring physician receives from the person or entity with whom the
physician has a direct financial relationship aggregate compensation that varies with, or otherwise
takes into account, the volume or value of the physicians’ referrals to, or other business generated
by the referring physician for, the entity furnishing the DHS; and (iii) the entity furnishing the
DHS has knowledge of the fact that the referring physician (or immediate family member) receives
aggregate compensation that varies with, or takes into account, the volume or value of referrals or
other business generated by the referring physician for the entity furnishing DHS. See 42 C.F.R.
§ 411.354(c)(2) (2014).
120. For purposes of the Stark Law, a “referral” includes any request by a physician for,
or ordering of, or the certifying or recertifying of the need for, any DHS for which Medicare
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 30 of 154 PageID #: 913
31
payment may be made, including a request for a consultation with another physician and any test
or procedure ordered by or to be performed by (or under the supervision of) that other physician,
but does not include any DHS personally performed by the referring physician. 42 U.S.C.
§ 1395nn(h)(5); 42 C.F.R. § 411.351 (2014).
121. “Other business generated” means “any other business generated by the referring
physician, including other Federal and private pay business.” 66 Fed. Reg. 856, 877 (Jan. 4, 2001).
122. Compensation is “deemed not to take into account ‘other business generated
between the parties,’ provided that the compensation is fair market value for items and services
actually provided and does not vary during the course of the compensation arrangement in any
manner that takes into account referrals or other business generated by the referring physician,
including private pay healthcare business. . . .” 42 C.F.R. § 411.354(d)(3) (2014).
123. The Stark Law and its companion regulations set forth exceptions for certain
financial relationships that meet specific enumerated requirements. The Stark Law’s exceptions
operate as affirmative defenses to alleged violations of the statute. Once it has been shown that a
party submitting Medicare claims has a financial relationship with a referring physician, the
defendant bears the burden of demonstrating that the relationship meets all of the requirements of
an applicable statutory or regulatory exception. See, e.g., United States ex rel. Drakeford v. Tuomey
Healthcare Sys., Inc., 675 F.3d 394, 405 (4th Cir. 2012).
124. The Stark Law and its implementing regulations contain exceptions for certain
compensation arrangements, including “personal service arrangements” and “indirect
compensation arrangements.”
125. To qualify for the Stark Law’s exception for personal service arrangements, a
compensation arrangement must meet, inter alia, the following statutory requirements: the
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 31 of 154 PageID #: 914
32
compensation (A) is set in advance, (B) does not exceed fair market value, and (C) is not
determined in a manner that takes into account the volume or value of any referrals or other
business generated between the parties, except for compensation received pursuant to a “physician
incentive plan” as defined by the Stark Law. See 42 U.S.C. § 1395nn(e)(3)(A); see also 42 C.F.R.
§ 411.357(d) (2014). A “physician incentive plan” under § 1395nn(e)(3) is narrowly defined and
only applies to personal service arrangements that “may directly or indirectly have the effect of
reducing or limiting services provided with respect to individuals enrolled with the entity.” 42
U.S.C. § 1395nn(e)(3)(B)(ii).
126. To qualify for the Stark Law’s exception for indirect compensation arrangements,
the following requirements, inter alia, must be satisfied: (A) the compensation received by the
referring physician is fair market value for items and services actually provided by the physician,
(B) the physician’s compensation is not determined in any manner that takes into account the
volume or value of referrals or other business generated by the referring physician for the DHS
entity, (C) the compensation is for identifiable services, and the arrangement is commercially
reasonable even in the absence of referrals to the entity, and (D) the arrangement does not violate
the AKS. See 42 C.F.R. § 411.357(p) (2014).
127. The Stark Law is a strict liability statute, with no scienter element. Those who
knowingly submit or cause to be submitted claims to Medicare in violation of the Stark Law also
violate the FCA. A knowing violation of the Stark Law also may result in exclusion from federal
healthcare programs. 42 U.S.C. §§ 1395nn(g)(3), 1320a-7a(a).
VII. LABORATORY TESTING OVERVIEW
128. Clinical laboratory services involve the “examination of materials derived from the
human body for the diagnosis, prevention, or treatment of a disease or assessment of a medical
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 32 of 154 PageID #: 915
33
condition.” Medicare Benefit Policy Manual (MBPM), Pub. 100-02, Ch. 15, § 80.1, available at
http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c15.pdf.
129. Pursuant to Medicare regulations, (1) laboratory tests must be ordered by the
physician treating the patient for the treatment of a specific illness or injury; (2) laboratory test
orders that are not individualized to patient need, or for which the need is not documented in the
medical record, are not covered services; and (3) claims for laboratory services that do not meet
these requirements are ineligible for payment. See 42 C.F.R. § 410.32.
130. All diagnostic laboratory tests “must be ordered by the physician who is treating
the beneficiary, that is, the physician who furnishes a consultation or treats a beneficiary for a
specific medical problem and who uses the results in the management of the beneficiary’s specific
medical problem. Tests not ordered by the physician who is treating the beneficiary are not
reasonable and necessary.” 42 C.F.R. § 410.32(a).
131. A laboratory test order is “a communication from the treating physician/practitioner
requesting that a diagnostic test be performed for a beneficiary.” MBPM, Ch. 15, § 80.6.1.
Medicare requires that an ordering physician “must clearly document, in the medical record, his
or her intent that the test be performed.” Id.
132. Clinical laboratory services must be used promptly by the physician who is treating
the beneficiary as described in 42 C.F.R. § 410.32(a). See MBPM, Ch. 15, § 80.1.
133. Medicare requires proper and complete documentation of laboratory services
rendered to beneficiaries. In particular, the Medicare statute provides that:
No payment shall be made to any provider of services or other person under this
part unless there has been furnished such information as may be necessary in order
to determine the amounts due such provider or other person under this part for the
period with respect to which the amounts are being paid or for any prior period.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 33 of 154 PageID #: 916
34
42 U.S.C. § 1395l(e); see also 42 U.S.C. § 1395u(c)(2)(B)(i) (“The term ‘clean claim’ means a
claim that has no defect or impropriety (including any lack of any required substantiating
documentation) . . . .”).
134. A laboratory’s claim for a service is ineligible for payment if there is not sufficient
documentation in the patient’s medical record to establish that the service was reasonable and
necessary. 42 C.F.R. § 410.32(d)(3).
135. Medicare regulations allow laboratories to request documentation from physicians
regarding medical necessity:
Medical necessity. The entity submitting the claim may request additional
diagnostic and other medical information from the ordering physician or
nonphysician practitioner to document that the services it bills are reasonable and
necessary.
42 C.F.R. § 410.32(d)(3)(iii).
136. Likewise, under the Texas Medicaid program, services must be individualized to
the medical needs of each patient; providers must maintain appropriate documentation for each
beneficiary, substantiating the need for services, including all findings and information supporting
medical necessity, and detailing all treatment provided. For laboratory services or tests to be
covered by Texas Medicaid, those services must be ordered by a professional practitioner within
the scope of his or her practice.
137. Similarly, TRICARE covers laboratory tests only if the tests are “medically or
psychologically necessary” and “required in the diagnosis and treatment of illness or injury.” 32
CFR § 199.4(a)(1). TRICARE will not cover tests that are “not related to a specific illness or injury
or a definitive set of symptoms.” Id. at § 199.4(g)(2).
138. As noted above, TRICARE regulations provide that TRICARE may deny payment
in “abuse situations.” 32 C.F.R. § 199.9(b). The regulations expressly include as examples of
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 34 of 154 PageID #: 917

35
“abuse or possible abuse situations” the following: (i) “a battery of diagnostic tests are given
when, based on the diagnosis, fewer tests were needed,” and (ii) “[f]ailure to maintain adequate
medical or financial records.” Id.
LABORATORY FRAUD SCHEMES
139. Laboratory executives and employees in Texas conspired with hospital executives
and employees, recruiters, and healthcare providers (HCPs), among others, to pay kickbacks to
HCPs to induce their referrals of laboratory testing, even when medically unnecessary. As part of
the conspiracy, LRH falsely represented to federal healthcare programs that the beneficiaries were
hospital outpatients, in order to fraudulently secure higher reimbursements.
I. LITTLE RIVER HOSPITAL FRAUD SCHEMES
A. LRH Submitted False Outpatient Claims to Receive Higher Reimbursement
1. Reimbursement to CAHs
140. To ensure that Medicare beneficiaries in rural communities can access necessary
hospital care, Congress authorized favorable Medicare reimbursements for hospitals certified by
CMS as critical access hospitals (CAHs). Balanced Budget Act of 1997, P.L. No. 105-33 § 4201.
141. To be certified as a CAH, hospitals participating in Medicare generally must,
among other things, have 25 or fewer inpatient beds, provide emergency services 24 hours per day,
and be located in underserved rural areas some distance from other hospitals or CAHs. 42 C.F.R.
§§ 485.610, 485.618, 485.620.
142. A hospital certified as a CAH is eligible to receive favorable Medicare
reimbursements, generally being paid 101 percent of reasonable costs for most inpatient and
outpatient services provided to Medicare beneficiaries. 42 U.S.C. § 1395m(g). The cost-based
payments to CAHs generally are much higher than the predetermined rates that Medicare pays
acute care hospitals (non-CAHs) and laboratories for the same services.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 35 of 154 PageID #: 918
36
143. Because Medicare’s favorable reimbursement to CAHs is meant to ensure access
to care by those in rural communities, a CAH is not eligible for cost-based reimbursement for
services provided to individuals who are neither inpatients nor outpatients of the CAH, i.e., non-
patients of the hospital. See 42 C.F.R. § 413.70 (2015).
144. As relevant here, for outpatient clinical diagnostic laboratory services, Medicare
will pay 101 percent of reasonable costs to a CAH “only if [1] the individual is an outpatient of
the CAH” and [2] either “[t]he individual is receiving outpatient services in the CAH on the same
day the specimen is collected” or “[t]he specimen is collected by an employee of the CAH.” 42
C.F.R. § 413.70(b)(7)(iv) (2015). Although an individual Medicare beneficiary need not be
“physically present in the CAH at the time the specimen is collected,” the individual must be “an
outpatient of the CAH.” Id.
145. The CAH can bill for outpatient services only if the individual beneficiary [1] “has
not been admitted as an inpatient,” [2] “is registered on the hospital or CAH records as an
outpatient and [3] receives services (rather than supplies alone) directly from the hospital or CAH.”
42 C.F.R. § 410.2.
146. If a Medicare beneficiary is neither an inpatient nor an outpatient of the CAH, then
reimbursement for the non-patient’s clinical diagnostic laboratory tests is based on the Medicare
clinical laboratory fee schedule (CLFS). 42 C.F.R. § 413.70(b)(7)(vi) (2015).
2. LRH Submitted False Outpatient Claims for Non-Patients of LRH
147. LRH was a CAH headquartered in Rockdale, Texas (population under 6,000).
148. LRH received cost-plus payments when it submitted hospital outpatient claims to
Medicare for laboratory testing. Such cost-plus payments significantly exceeded the payments
available under the CLFS for claims to Medicare for laboratory testing on non-patients of LRH.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 36 of 154 PageID #: 919

37
149. For example, the chart below includes laboratory tests in a panel that Elizabeth
Seymour, M.D., of Denton, Texas, ordered from LRH on or about August 1, 2016 for a Medicare
beneficiary in return for MSO kickbacks. LRH submitted claims for the tests to Medicare, falsely
representing that the services were provided to LRH outpatients. The chart lists the amounts that
Medicare paid to LRH for purported outpatient services. In comparison, the chart lists the
corresponding Medicare payment amount in 2016 in Texas under the CLFS.
CPT
Code
CPT Description
LRH
Payment
CLFS
Payment
80053
Blood test, comprehensive group of blood chemicals
$99.06
$14.39
80061
Blood test, lipids (cholesterol and triglycerides)
$112.84
$18.24
82172
Apolipoprotein level
$94.59
$21.11
82306
Vitamin D-3 level
$125.13
$37.02
82533
Cortisol (hormone) measurement, total
$54.00
$22.21
82542
Chemical analysis using chromatography technique
$91.53
$24.60
82542
Chemical analysis using chromatography technique
$45.77
$24.60
82607
Cyanocobalamin (vitamin B-12) level
$70.76
$20.54
82610
Cystatin C (enzyme inhibitor) level
$41.34
$18.52
82627
Dehydroepiandrosterone (DHEA-S) hormone level
$67.78
$30.29
82664
Electrophoresis, laboratory testing technique
$79.32
$35.62
82670
Measurement of total estradiol (hormone)
$94.22
$38.06
82725
Fatty acids measurement
$40.59
$18.13
82747
Folic acid level, RBC
$125.50
$23.55
82777
Galectin-3 level
$67.03
$29.96
83001
Gonadotropin, follicle stimulating (reproductive hormone) level
$58.09
$25.31
83002
Gonadotropin, luteinizing (reproductive hormone) level
$66.29
$25.22
83090
Homocysteine (amino acid) level
$109.11
$22.98
83525
Insulin measurement, total
$46.92
$15.57
83698
Lipoprotein-associated phospholipase A2 (enzyme) level
$103.15
$46.24
83704
Lipoprotein level, quantitation of lipoprotein particle number(s)
$197.37
$42.98
83789
Mass spectrometry (laboratory testing method)
$24.58
$24.60
83876
Myeloperoxidase (white blood cell enzyme) measurement
$103.15
$46.24
83880
Natriuretic peptide (heart and blood vessel protein) level
$103.15
$46.24
83921
Organic acid level
$121.40
$22.41
84140
Pregnenolone (reproductive hormone) level
$36.70
$28.16
84144
Progesterone (reproductive hormone) level
$63.31
$28.42
84206
Proinsulin (pancreatic hormone) level
$54.00
$24.26
84311
Chemical analysis using spectrophotometry (light)
$10.80
$9.52
84378
Carbohydrate analysis, single quantitative
$84.16
$3.92
84403
Testosterone (hormone) level, total
$101.67
$35.17
84443
Blood test, thyroid stimulating hormone (TSH)
$128.85
$22.89
84481
Thyroid hormone, T3 measurement, free
$61.07
$23.07
84482
Thyroid hormone, T3 measurement, reverse
$23.46
$10.48
84550
Uric acid level, blood
$54.00
$6.16
84681
C-peptide (protein) level
$27.56
$28.35
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 37 of 154 PageID #: 920

38
CPT
Code
CPT Description
LRH
Payment
CLFS
Payment
85025
Complete blood cell count (red cells, white blood cell, platelets),
automated test and automated differential white blood cell count
$45.81 $10.59
85385
Fibrinogen (factor 1) antigen detection
$25.70
$11.57
86141
Measurement C-reactive protein for detection of infection or
inflammation, high sensitivity
$102.41 $17.63
86341
Islet cell (pancreas) antibody measurement
$52.51
$23.57
86376
Microsomal antibodies (autoantibody) measurement
$61.82
$19.82
86800
Thyroglobulin (thyroid protein) antibody measurement
$40.59
$21.67
Total:
$3,117.09
$1,019.88
150. By billing LRH’s laboratory claims for non-patient tests (under the CLFS) as if they
were outpatient tests (cost-plus reimbursement), LRH inflated its laboratory claims for this patient
by over $2,000 to receive more than triple the CLFS amount.
151. CAHs also receive higher reimbursement when they submit claims for other
diagnostic services, such as sleep studies or electroencephalogram (EEG) tests, performed on
hospital outpatients.
152. The higher reimbursement Medicare pays to CAHs like LRH is meant to ensure
that patients in rural communities, such as in Rockdale, Texas, can access necessary hospital care.
153. Rather than focus on providing necessary hospital care to the community, LRH
CEO Madison, LRH CFO Borgfeld, and their co-conspirators agreed to and implemented a plan
to defraud federal healthcare programs by funneling claims for diagnostic services, including
laboratory tests, for hospital non-patients through LRH for higher reimbursement.
154. Madison and Borgfeld agreed with two laboratories, BHD and THD, and their
executives to bill federal healthcare programs for laboratory testing performed by BHD and THD.
Madison and Borgfeld agreed to pay numerous recruiters to recommend and arrange for providers
throughout Texas to order laboratory testing through LRH for beneficiaries who were neither LRH
inpatients nor LRH outpatients.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 38 of 154 PageID #: 921
39
155. To further the fraud scheme, Madison and Borgfeld agreed to pay phlebotomists or
medical staff located in the offices of primary care providers (PCPs) throughout Texas to draw the
beneficiaries’ blood. Often, these phlebotomists had previously worked for the PCP’s office, BHD,
or THD. As examples, LRH paid Lacrimioara Hurgoiu, who was already working in Dr. Annie
Varughese’s office as a registered nurse, $18 per hour for 30-40 hours a week to draw blood for
laboratory tests that Varughese referred to LRH. LRH paid Tracy Tompkins, who was already
working in Dr. Elizabeth Seymour’s office as a phlebotomist, $19 per hour for 30-40 hours a week
to draw blood for laboratory tests that Seymour referred to LRH. Borgfeld often signed the
“Clinical Specialist Services” Agreements on LRH’s behalf for the LRH-paid phlebotomists.
156. Pursuant to the scheme agreed to by Madison and Borgfeld, LRH employees and
recruiters directed the phlebotomists located in PCPs’ offices to create false hospital registration
records identifying the PCPs’ patients as LRH outpatients for purposes of billing laboratory tests
performed by BHD or THD.
157. LRH’s claims to federal healthcare programs for laboratory testing falsely
represented, among other things, that the tests were for LRH outpatients, when in fact the
beneficiaries were non-patients of LRH.
158. Many of the beneficiaries were more than 100 miles away from LRH and had never
even heard of the hospital, much less ever been a patient there.
159. Nearly all of the providers who ordered BHD or THD laboratory testing through
LRH had no admitting privileges at LRH, had never practiced at LRH, had never referred to LRH
before participating in the MSO kickback scheme, and had never even visited LRH’s Rockdale
hospital.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 39 of 154 PageID #: 922
40
160. To induce providers’ referrals for diagnostic services reimbursed by federal
healthcare programs, including laboratory tests, Madison, Borgfeld, and their co-conspirators
agreed to a scheme to pay thousands of dollars to providers who referred to LRH, while disguising
the payments as purported MSO investment distributions.
B. LRH’s MSO Kickback Scheme
161. In or about 2014, Madison and Borgfeld developed a “growth plan” to take
advantage of LRH’s “higher reimbursement levels and government subsidies.” Aware that as a
CAH, LRH received “cost based reimbursement which enhances financial performance for rural
hospitals,” Madison and Borgfeld developed a plan for “immediate near term significant growth.”
162. To further their plan, Madison and Borgfeld sought to increase referrals for
toxicology laboratory testing. Their goal was to “engage as many toxicology practices as
reasonably possible” to refer to LRH. Madison and Borgfeld sought to secure physician referrals
for toxicology testing by offering to “incentivize physicians to become part of [LRH’s] business
model.” Madison and Borgfeld understood that LRH would have the financial wherewithal to offer
incentives for physicians to refer toxicology testing to LRH because LRH “receives better payment
rates than private practices” would receive if they billed insurers.
163. In 2014, Madison and Borgfeld began implementing their plan to incentivize HCPs
to refer toxicology testing to LRH. At their direction, LRH began entering into contracts to pay
commissions to recruiters, who in turn would pay financial incentives to HCPs to induce their
referrals. On or about August 1, 2014, LRH agreed to pay independent contractor S&G, a company
owned and operated by Scott and Ginny Jacobs.
164. Pursuant to the toxicology scheme developed by Madison and Borgfeld, urine
specimens were collected in the referring HCP’s office, a toxicology laboratory ran the tests (for
a fee paid by LRH), and LRH or a contracted billing company submitted the claims to insurers on
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 40 of 154 PageID #: 923
41
LRH’s behalf. As part of their scheme, LRH paid S&G to recruit HCPs to order toxicology tests
from LRH, and S&G paid kickbacks to the referring HCPs to order toxicology tests from LRH.
Parties to the toxicology scheme understood that the payments to HCPs were kickbacks, merely
disguised as investment distributions.
165. In 2015, Madison and Borgfeld expanded the fraud scheme to include blood testing.
Madison and Borgfeld, on behalf of LRH, agreed to pay per-test fees to BHD and later THD to
run blood tests for LRH. To gain referrals, LRH paid recruiters to arrange for and recommend
HCPs’ referrals for blood testing, and the recruiters kicked back some of those payments to the
referring HCPs, while disguising the payments to HCPs as investment distributions from an MSO.
The MSO-incentivized HCPs ordered BHD and/or THD testing from LRH.
166. In the toxicology and blood laboratory testing schemes, LRH billed the tests to
federal healthcare programs as outpatient services, falsely representing that (a) the claims did not
result from AKS or Stark Law violations; (b) the tests were for LRH outpatients, when in fact the
tests were for persons who were not patients at LRH at all; and (c) the claims were for reasonable
and necessary services.
C. LRH Funded the MSO Kickbacks to HCPs
167. LRH funded the MSO kickbacks to HCPs, with the knowledge and approval of
Madison and Borgfeld. LRH paid recruiters to generate commercial and federal laboratory testing
referrals; the recruiters transferred a portion of the funds to the recruiters’ MSO entities; the MSOs
paid the referring HCPs to induce their referrals to LRH; and LRH submitted the resulting claims
to Medicare, Medicaid, and TRICARE.
168. With Madison and Borgfeld’s knowledge and approval, LRH agreed to fund the
MSO kickbacks by paying volume-based commissions to six sets of recruiters to arrange for and
recommend referrals to LRH for toxicology and/or blood testing: (a) S&G; (b) Jacobs Marketing,
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 41 of 154 PageID #: 924
42
a corporation owned and operated by Scott and Ginny Jacobs; (c) Next Level, a company owned
and operated by Ruben Marioni and Jordan Perkins; (d) LGRB, a company owned and operated
by Stanley Jones, Jeffrey Parnell, and Thomas Gray Hardaway; (e) Exit Therapy LLC (Exit
Therapy), a company Robert O’Neal established in his wife’s name; and (f) APC, a company
owned and operated by Todd Hickman and O’Neal.
169. To fund the MSO kickbacks to HCPs, as Madison and Borgfeld knew and
approved, LRH paid over $18.5 million to recruiters during the MSO kickback scheme, as follows:
a. Over $1.95 million to S&G (since in or about August 2014);
b. Over $3.4 million to Jacobs Marketing (since in or about March 2015);
c. Over $5.9 million to Next Level (since in or about March 2015);
d. Over $3.1 million to LGRB (since in or about May 2015);
e. Over $280,000 to Exit Therapy (since in or about May 2015); and
f. Over $4.1 million to APC (since in or about July 2015).
D. LRH’s Recruiters Paid the MSO Kickbacks to HCPs
170. Madison and Borgfeld understood that few HCPs would order toxicology or blood
tests from a CAH headquartered in Rockdale, Texas without a financial incentive to do so.
171. In their discussions with recruiters, Madison and Borgfeld understood that the
recruiters would offer and pay money to HCPs to induce them to order laboratory testing from
LRH. Madison and Borgfeld understood that the recruiters would attempt to disguise the kickback
payments to referring HCPs as purported fees from an MSO. Madison and Borgfeld met and
corresponded with the recruiters and agreed to the MSO kickback scheme.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 42 of 154 PageID #: 925

43
Jacobs and Jacobs
172. LRH paid millions of dollars to S&G and Jacobs Marketing to arrange for and
recommend that HCPs refer to LRH for toxicology and blood testing, respectively, and to fund the
MSO kickbacks to HCPs.
173. To induce the HCP referrals to LRH, Scott and Ginny Jacobs paid referring HCPs
through two MSOs they owned and operated, North Houston MSO Group, Inc. (North Houston
MSO) and Tomball Medical Management, Inc. (Tomball MSO) (collectively, Jacobs’ MSOs).
174. Scott and Ginny Jacobs transferred funds that S&G and Jacobs Marketing received
from LRH to the Jacobs’ MSOs by means of direct and indirect transfers to the Jacobs’ MSOs
through other corporate entities owned and operated by Scott and Ginny Jacobs, including
Strategic Medical Solutions, Inc. and Texas Premier Management Group LLC.
175. The Jacobs’ MSOs paid over $1.1 million to the following HCPs to induce their
referrals to LRH:
HCP
MSO
MSO Payments
Alan Tran North Houston $14,253
Amrit Thandi North Houston $20,500
Angela Mosley-Nunnery
(Physician A)
North Houston; Tomball $83,250
Annie Varughese North Houston $8,000
Asif Ali North Houston $2,000
Butch Martin North Houston $17,650
Candice DeMattia
North Houston; Tomball
$103,503
David Le North Houston; Tomball $54,536
E.P. Descant North Houston; Tomball $128,233
Earl F. Martin
North Houston
$17,650
Earl “Butch” Martin North Houston $17,650
George Murillo North Houston; Tomball $81,633
Jason DeMattia North Houston; Tomball $158,071
Mark Le
North Houston; Tomball
$28,950
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 43 of 154 PageID #: 926

44
HCP MSO MSO Payments
Michael Casagrande North Houston; Tomball $99,533
Michael Diteresa
North Houston
$4,179
Michael Whiteley North Houston; Tomball $77,883
Michelle Legall North Houston; Tomball $20,000
Randall Walker
North Houston; Tomball
$30,449
Richard Le North Houston; Tomball $20,500
Steven Chon North Houston $27,600
Tamar Brionez Tomball $93,024
176. Scott Jacobs and recruiter and referring HCP Jason DeMattia pitched the Jacobs’
MSOs to HCPs, including groups of HCPs. Only HCPs who referred to LRH were allowed to
participate and remain in the Jacobs’ MSOs, and the only source of revenue for the Jacobs’ MSOs
came from the referrals or other business generated by the HCPs in the MSOs. In their sales pitches
to HCPs, Scott Jacobs and DeMattia described the Jacobs’ MSOs as an opportunity for HCPs to
share in the profits generated by the HCPs’ referrals of toxicology and blood testing to LRH.
177. To disguise the kickbacks, Scott and Ginny Jacobs used purported “investment”
documentation for the Jacobs’ MSOs. While HCPs purported to invest in the Jacobs’ MSOs, the
MSOs’ payments to HCPs were not based on the returns from any genuine investment. Instead,
the Jacobs’ MSOs’ payments to HCPs were simply profits shared with HCPs based on the HCPs’
referrals to LRH. After agreeing to participate in a Jacobs’ MSO and referring laboratory testing
to LRH, the Jacobs’ MSOs often paid the HCP more in the first month of MSO distributions than
the HCP had invested. That is, HCPs in the Jacobs’ MSOs often received one or more MSO
payments before Scott or Ginny Jacobs deposited the purported investment check.
178. For example, in or about April 2016, Physician A, of Kingwood, Texas, agreed to
participate in the Tomball MSO, provided Scott Jacobs with a purported $2,000 investment check,
and began referring to LRH for laboratory testing.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 44 of 154 PageID #: 927
45
179. Physician A had no admitting privileges at LRH, had never practiced at LRH, had
never referred to LRH before joining the Tomball MSO kickback scheme in or about April 2016,
and had never even visited LRH’s Rockdale hospital.
180. Scott and Ginny Jacobs did not deposit Physician A’s check until on or about July
21, 2016. By that date, Physician A had made dozens of referrals to LRH for laboratory testing. In
addition, Tomball MSO already had paid Physician A two checks of $2,000 each on or about July
5, 2016, totaling twice the amount purportedly invested weeks later. On or about July 22, 2016,
the day after her purported investment check was deposited, Tomball MSO paid Physician A
another $2,000.
181. From in or about July 2016 to July 2017, Tomball MSO paid Physician A $37,500
in MSO payments, for a 17,000% return on investment.
182. As the Tomball MSO was winding down in early 2017, Scott Jacobs and DeMattia
convinced Physician A to join the North Houston MSO in or about January 2017. Physician A
agreed to participate in the North Houston MSO and provided Scott Jacobs with a purported
investment check for $4,000.
183. Again, Scott and Ginny Jacobs waited to deposit Physician A’s check until their
MSO had already paid Physician A more than the purported investment. North Houston MSO
deposited Physician A’s check on or about March 21, 2017. By that date, North Houston MSO
already had paid Physician A $8,000—double the purported investment amount—on or about
February 13, 2017.
184. From in or about February 2017 to February 2018, North Houston MSO paid
Physician A $51,750 in MSO payments, for an 11,937% return on investment.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 45 of 154 PageID #: 928

46
185. From in or about April 2016 to December 2017, during the Tomball MSO and
North Houston MSO kickback schemes, Physician A referred to LRH hundreds of tests payable
by federal healthcare programs. LRH submitted those claims to federal healthcare programs as
purported outpatient services, and Medicare paid over $360,000 to LRH. Examples of those claims
are included in Exhibit A hereto.
Marioni and Perkins
186. LRH paid millions of dollars to Next Level to arrange for and recommend that
HCPs refer to LRH for toxicology and blood testing and to fund the MSO kickbacks to HCPs.
187. Marioni and Perkins transferred the funds that Next Level received from LRH to
numerous MSOs that they owned and operated, including SYNRG Partners LLC (SYNRG) and
Permian Partners LLC (Permian) (collectively, “Next Level MSOs”).
188. Through the Next Level MSOs, Marioni and Perkins paid referring HCPs to induce
their referrals to LRH. The below chart summarizes Next Level MSOs’ payments of over $685,000
to the following HCPs to induce their referrals to LRH:
HCP
MSO
MSO Payments
Ambreen Sharaf
SYNRG
$22,528
Annie Varughese SYNRG $70,664
Ashley Chin SYNRG $22,528
Cuong Trinh
SYNRG
$22,528
Jaspaul Bhangoo Permian $13,000
Kozhaya Sokhon SYNRG $47,056
Murtaza Mussaji SYNRG; Permian $52,056
Nina Pham
SYNRG
$22,528
Parul Shah SYNRG $47,056
Rakesh Patel SYNRG $117,640
Saira Hirani
SYNRG
$22,528
Shane Simpson SYNRG $11,764
Thien Nguyen SYNRG $22,528
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 46 of 154 PageID #: 929

47
HCP MSO MSO Payments
Tommy Pham SYNRG; Permian $52,056
Trang Trinh
SYNRG
$117,640
Victoria Do SYNRG $22,528
189. To disguise the kickbacks, Marioni and Perkins used purported “investment”
documentation for the Next Level MSOs. Only HCPs who referred to LRH were allowed to
participate and remain in the Next Level MSOs, and the only source of revenue for the Next Level
MSOs came from the referrals or other business generated by the HCPs in the MSOs. While HCPs
purported to invest in the Next Level MSOs, the MSOs’ payments to HCPs were not based on the
returns from any genuine investment. Instead, the Next Level MSOs’ payments to HCPs were
profits shared with HCPs based on the HCPs’ referrals to LRH.
190. In or about November 2015, Next Level created a written presentation about the
company based on the information that Marioni and Perkins typically provided to HCPs in their
sales pitch. Next Level noted that the MSO would “help[]” HCPs by offering them a “Low risk,
high return” opportunity. Next Level stated that it “is focused on advanced lipid testing provided
through [BHD],” “[b]illing is processed through [LRH],” and Next Level MSO would receive a
share of the monies collected. Next Level explained that the “Buy in amount is $1500 per percent”
ownership, and in an “MSO with high participation ~1600 samples per month” (i.e., a high volume
of referrals to LRH), a “[p]hysician with 1% ownership receives $11,520/month.”
191. That is, Next Level was offering to pay HCPs $11,520 per month—$138,240 per
year—if the HCPs made a nominal $1,500 contribution and had “high participation” in the scheme
to refer laboratory testing to LRH. As its summary indicated, the Next Level MSOs were not a
genuine investment opportunity; they were a profit-sharing arrangement to pay HCPs a share of
the revenue generated by their referrals.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 47 of 154 PageID #: 930
48
192. In describing the HCP sign up process, Next Level highlighted the MSO’s profit-
sharing motive. First, “[p]hysician signs paperwork to begin 30-45 day period of evaluation period
(monies accrue in MSO account).” During the evaluation period, “[LRH] will place a phlebotomist
[in the physician’s office] or hire physician’s current phlebotomist.” “At the end of evaluation
period shares are offered to physician.” Only after the month-plus evaluation period, once Next
Level had evidence of the volume of a HCP’s referrals to LRH, would Next Level place a referring
HCP in a Next Level MSO with other HCPs of similar referring volume. Finally, to disguise the
kickbacks, “[c]ontracts are signed and checks are collected” by Next Level.
193. Given the volume of LRH referrals that the Next Level MSOs generated, LRH
assured Marioni and Perkins, in an email copying Madison and Borgfeld, that “YOU ARE THE
‘A TEAM,’” claiming that another “recruiter group” for LRH (S&G and Jacobs Marketing) had
“less than a handful of friends that they added into their MSO that practice outside Tomball.”
194. For example, in or about September 2015, Next Level recruited Annie Varughese,
M.D. (Physician B), of The Woodlands, Texas, to refer laboratory testing to LRH. To induce
Physician B’s referrals to LRH, Perkins and Marioni offered to pay her MSO kickbacks through
SYNRG MSO. Physician B agreed to participate in the kickback scheme and began referring
laboratory tests for federal healthcare beneficiaries to LRH on or about October 9, 2015.
195. Before Next Level offered to pay Physician B MSO kickbacks through SYNRG
MSO, Physician B had never previously ordered laboratory tests from LRH. Physician B did not
have admitting privileges at LRH. Physician B had never practiced at LRH. And Physician B had
never referred any patient to LRH before agreeing to participate in the MSO kickback scheme.
Indeed, Physician B’s medical practice in The Woodlands, Texas was over 100 miles away from
LRH’s Rockdale, Texas facility.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 48 of 154 PageID #: 931
49
196. During the period of in or about September 2015 to at least July 2016, SYNRG
MSO paid Physician B over $70,000 in MSO kickbacks, a return of over 1,455% based on her
purported investment of $4,500.
197. From in or about October 2015 to September 2016, during Physician B’s
participation in the SYNRG MSO kickback scheme, Physician B referred to LRH hundreds of
tests payable by federal healthcare programs. LRH submitted those claims as purported outpatient
services to federal programs, including Medicare, which paid over $600,000 to LRH. Examples of
those claims are included in Exhibit A hereto.
198. As another example, in or about April 2015, Next Level recruited Trang Trinh,
M.D. (Physician C), of Katy, Texas, to refer laboratory testing to LRH. To induce Physician C’s
referrals to LRH, Perkins and Marioni offered to pay MSO kickbacks to Physician C through
SYNRG MSO. Physician C agreed to participate in the kickback scheme and began referring
laboratory tests for federal healthcare beneficiaries to LRH on or about April 27, 2015.
199. Before Next Level offered to pay MSO kickbacks to Physician C through SYNRG
MSO, Physician C had never previously ordered laboratory tests from LRH. Physician C did not
have admitting privileges at LRH. Physician C had never practiced at LRH. And Physician C had
never referred any patient to LRH before agreeing to participate in the MSO kickback scheme.
Indeed, Physician C’s medical practice in Katy, Texas was over 100 miles away from LRH’s
Rockdale, Texas facility.
200. During the period of in or about April 2015 to June 2016, SYNRG MSO paid
Physician C over $117,000 in MSO kickbacks, a return of over 1,460% based on her purported
investment of $7,500.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 49 of 154 PageID #: 932

50
201. From in or about April 2015 to June 2016, during Physician C’s participation in the
SYNRG MSO kickback scheme, Physician C referred to LRH hundreds of tests payable by federal
healthcare programs. LRH submitted those claims as purported outpatient services to federal
programs, including Medicare, which paid over $200,000 to LRH. Examples of those claims are
included in Exhibit A hereto.
O’Neal and Hickman
202. In early 2015, Madison described to O’Neal the MSO model that LRH and its
recruiters were using to provide financial incentives to HCPs to order testing from LRH. In or
about April 2015, Madison and Borgfeld offered O’Neal the opportunity to be paid by LRH for
recruiting HCPs to order diagnostic services from LRH.
203. In or about May 2015, O’Neal agreed to be paid through Exit Therapy to arrange
for and recommend HCP referrals to LRH. Like LRH’s other recruiters, Madison and Borgfeld
understood that O’Neal would kickback a portion of the Exit Therapy payments to referring HCPs,
in the form of MSO payments, to induce the HCPs’ referrals to LRH.
204. As part of the arrangement, Exit Therapy transferred funds to O’Neal’s company,
Quick Diagnostics, Inc. (Quick MSO), and Quick MSO paid HCPs who referred to LRH.
205. O’Neal partnered with Kash, Howard, and Gonzales to recruit HCPs to refer to
LRH in return for payments from Quick MSO. Kash, Howard, and Gonzales each had worked as
sales representatives in Texas and knew numerous HCPs in Texas. Kash, Howard, and Gonzales
spoke with HCPs to offer MSO payments to induce the HCPs’ referrals to LRH. Kash, Howard,
and Gonzales provided Quick MSO documents to prospective HCP participants, arranged for and
recommended that the HCPs order laboratory tests through LRH, and distributed payment checks
to referring HCPs.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 50 of 154 PageID #: 933
51
206. In or about August 2015, Hickman joined the MSO kickback scheme. To further
that scheme, Hickman founded, owned, and operated numerous corporate entities. He created APC
to receive payments from LRH and make payments to an MSO, to Kash, to Hickman, and to
another company Hickman created, APM.
207. Hickman created APM to receive payments from APC to pay himself and others.
208. Hickman created Ascend MSO of TX LLC (Ascend MSO) to receive payments
from APC, to pay recruiters like Gonzales, and to pay HCPs who referred to LRH.
209. In light of its agreement with APC, LRH asked O’Neal to “mov[e] the Exit Therapy
doctors under the Ascend contract as of December 1, 2015.” LRH then terminated its agreement
with Exit Therapy, while maintaining its contract with APC.
210. In or about August 2015, Ascend MSO recruiters Kash, Gonzales, and Howard
began implementing the Ascend MSO kickback scheme, targeting HCPs, offering kickbacks, and
coordinating with BHD, THD, and their personnel.
211. To disguise the kickbacks, Hickman and O’Neal used purported “investment”
documentation for the Ascend MSO. Only HCPs who referred to LRH were allowed to participate
and remain in the Ascend MSO, and the only source of revenue for the Ascend MSO came from
the referrals or other business generated by the HCPs in the MSO. While HCPs purported to invest
in the Ascend MSO, the MSO’s payments to HCPs were not based on the returns from any genuine
investment. Instead, the Ascend MSO’s payments to HCPs were simply profits shared with HCPs
based on the HCPs’ referrals to LRH.
212. Ascend MSO’s marketing director summarized the financial inducements in a “pro
forma” sent to Kash, Howard, and Gonzales. The pro forma showed how much money HCPs could
make based on their referrals of diagnostic services, including laboratory testing. In the Ascend
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 51 of 154 PageID #: 934
52
MSO pro forma for a “[g]roup of 10 doctors,” HCP owners were told they would have “multiple
revenue streams,” and would receive a share of the revenue generated by their referrals for
toxicology testing, blood testing, EEG tests, sleep studies, and other diagnostic services.
213. In their sales pitch to HCPs, the Ascend MSO recruiters focused on the amount of
money that HCPs would receive.
214. For example, on or about October 24, 2015, Kash offered the MSO kickbacks to
Charles Evans, M.D., of Lufkin, Texas. The following day, the HCP told Kash that he was “80%
sold on this” proposal, but had a few questions. In describing Kash’s sales pitch, the HCP said that
it “sound[ed] like a get rich quick scam”—and a “risky one at that”—in which he “could make an
extra million dollars in one year only to go bust 2 years down the road by doing so.” The HCP said
he was “struggling with how I label this income from the MSO” as it “clearly is not for medical
services” provided to the MSO. The HCP warned Kash that “[t]he doctors that sign on what you
presented yesterday are going to be skeamers [sic] for get rich quick, and I fear they will be
ordering unnecessary tests that will get us investigated.”
215. In response, Kash promised the HCP to “alleviate all your concerns” about “any
unknown surprises” and said “we want a 10 year working relationship vs a 6 month fiasco.”
216. Later that day, the HCP told Kash he was “pretty sure you can count on our [THD]
business.” The HCP told Kash “this is all pending the hard sale [sic] with [my wife],” explaining
that “[w]e will need it presented in a way to her that makes it look like the patient will be in better
hands financially and quality and types of care than we have presently.” The HCP noted that for
the laboratory tests billed by the hospital, “[t]he size of these bills are outrageous,” the patients
will not want to pay the bills, and the hospital will not want to take a loss, so “[t]his only works if
the insurance companies take the brunt of things.”
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 52 of 154 PageID #: 935
53
217. As another example, in or about October 2015, Howard and Gonzales offered
Ascend MSO kickbacks to another doctor in this District, Hong Davis, M.D. (Physician D), of
Plano, Texas, to induce Physician D to order BHD tests through LRH. Before being offered the
kickbacks, Physician D had never referred to LRH, a hospital nearly 200 miles away in Rockdale,
Texas. After agreeing on or about October 20, 2015 to receive the Ascend MSO kickbacks,
Physician D began referring patients, including Medicare beneficiaries, to LRH for laboratory
testing.
218. Physician D provided Gonzales with a purported “investment” check of $1,000,
dated January 14, 2016, from her practice, Hong Davis, M.D. P.A., to Ascend MSO. In the “For”
line of the check, Physician D confirmed it was for the “Boston Heart Partnership.”
219. Physician D ordered BHD tests through LRH because of the money Howard and
Gonzales had offered her. After referring testing to LRH, Physician D repeatedly asked Gonzales
when she would be paid for her referrals. In February 2016, Physician D asked Gonzales,
“Expecting time to receive the payment check?” In April 2016, Physician D asked Gonzales, “I
trust you will have my check ready tomorrow?” The following day, Physician D complained to
Gonzales, “it sound [sic] very fishy and not right, look like we send you all the samples, not only
just to get nothing, but also lost $1,000.” Physician D pleaded, “I really wish you and Laura
[Howard] can tell me the truth, now, if you guys know it.” Physician D said, “I am not satisfied, I
have not see [sic] a dime and I have already lost $1,000!” Physician D noted that “for 5 months no
distribution, never heard of.” Physician D indicated she did not need O’Neal “to be how are you,
fine, and you person. I just need him to show me the number!”
220. On or about May 6, 2016, Physician D received a $5,000 check that Hickman
authorized and signed on behalf of Ascend MSO. About two weeks later, Physician D received a
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 53 of 154 PageID #: 936
54
$6,438 check that Hickman authorized and signed on behalf of Ascend MSO. In 2016, as
authorized by Hickman, Ascend MSO paid $54,871 to Physician D for her referrals to LRH, a
5,387% return on investment.
221. From in or about November 2015 to July 2016, during her participation in the
Ascend MSO kickback scheme, Physician D referred to LRH dozens of tests payable by federal
healthcare programs. LRH submitted those claims as purported outpatient services to federal
programs, including Medicare, which paid thousands of dollars to LRH. Example of those claims
are included in Exhibit A hereto.
222. As another example, in or about April 2016, APC recruited Elizabeth Seymour,
M.D. (Physician E), of Denton, Texas, to refer to LRH for laboratory testing. To induce Physician
E’s referrals to LRH, Gonzales offered to pay MSO kickbacks to Physician E through Ascend
MSO. Physician E agreed to participate in the kickback scheme, gave Gonzales a purported
investment check of $1,000, and began referring laboratory tests, including for federal healthcare
beneficiaries, to LRH on or about April 14, 2016.
223. Before Gonzales offered to pay MSO kickbacks to Physician E through Ascend
MSO, Physician E had never previously ordered laboratory tests from LRH. Physician E did not
have admitting privileges at LRH. Physician E had never practiced at LRH. And Physician E had
never referred any patient to LRH before agreeing to participate in the MSO kickback scheme.
Indeed, Physician E’s medical practice in Denton, Texas was over 180 miles away from LRH’s
Rockdale, Texas facility.
224. To eliminate any financial risk for Physician E, Hickman held Physician E’s
purported investment $1,000 check without depositing it until he and Gonzales had already
provided her with a much larger MSO payment check. Hickman did not deposit Physician E’s
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 54 of 154 PageID #: 937

55
check until on or about July 11, 2016. By that date, Physician E had made dozens of referrals to
LRH for laboratory testing, and Gonzales already had given Physician E a check dated July 6,
2016 for $5,000—five times the amount of Physician E’s purported investment.
225. Ascend MSO paid Physician E $49,000 in MSO kickbacks, a return of 4,800%
based on Physician E’s purported investment of $1,000.
226. From in or about April to August 2016, during her participation in the Ascend MSO
kickback scheme, Physician E referred to LRH hundreds of tests payable by federal healthcare
programs. LRH submitted those claims to federal healthcare programs as purported outpatient
services, and Medicare paid over $350,000 to LRH. Examples of those claims are included in the
chart in paragraph 149 above.
227. The HCPs who joined the Ascend MSO kickback scheme and referred laboratory
tests and other diagnostic services to LRH profited handsomely.
228. The below chart summarizes over $1.2 million in Ascend MSO payments from in
or about February 2016 to November 2017 to referring HCPs to induce their referrals to LRH:
HCP
MSO Payments
Azim Karim
$17,000.00
Bao Vinh Nguyen Phuc $55,870.84
Baxter Montgomery $78,070.84
Bruce Maniet
$50,870.84
Doyce Cartrett $36,000.00
Dung Hoy Nguyen and Dung Chi Nguyen $55,870.84
Elizabeth Seymour $49,000.00
Frederick Brown
$101,741.68
Heriberto Salinas $55,870.84
Hong Davis $55,870.84
Huy Chi Nguyen
$55,920.84
James Froelich III $50,870.84
Jill Taylor $78,070.84
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 55 of 154 PageID #: 938

56
HCP MSO Payments
Joseph Bolin $55,870.84
Muhammad Akram Khan
$55,870.84
Nicholas Aguilar $10,438.84
O. Michael Sprintig $10,000.00
Paul Gerstenberg
$101,741.68
Paul Worrell $54,000.00
Robert Hernandez $27,044.84
Robert Megna $34,000.00
Thuy Nguyen and Linh Ba Nguyen
$111,741.68
229. Ascend MSO owner Hickman and Ascend MSO recruiters Kash, Gonzales, and
Howard received hundreds of thousands of dollars for their actions in furtherance of the kickback
scheme.
230. At Hickman’s direction, APM paid Hickman’s company, Hickman Tax and
Retirement Advisors, $389,221.57 in 2016.
231. In an attempt to hide his role in the kickback scheme, Kash had his payments
funneled through a shell company named Tigerlily LLC, of which he was the beneficial owner. In
2016, APC paid Kash, through Tigerlily, a total of $191,334.
232. Howard also sought to conceal her role in the kickback scheme. Rather than receive
payments directly from an Ascend entity, Howard and Gonzales agreed that Gonzales’ company,
Zalegon Sales Associates LLC (Zalegon), would receive the payments, and that Gonzales would
share the proceeds with Howard. In 2016, Ascend MSO paid Zalegon $506,823.87.
233. As agreed with Howard, Gonzales deposited the checks Zalegon received from
Ascend MSO and withdrew cash to share with Howard. Approximately monthly, from May to
December 2016, Gonzales delivered to Howard the cash in a bag. Gonzales paid Howard about
$10,000 in cash per month, except for December 2016, when Gonzales paid Howard about $70,000
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 56 of 154 PageID #: 939

57
in cash. Each month, after Howard received the bag of cash from Gonzales, she placed it in the
safe in her home, with the cash still in the bag. In total, Gonzales paid Howard about $140,000 in
cash from Ascend MSO.
Jones, Parnell, and Hardaway
234. In 2015 and through at least June 2016, Parnell and Hardaway were BHD sales
representatives. With Jones, they owned and operated LGRB. LRH paid LGRB to recruit HCPs to
refer to LRH and to fund the MSO kickbacks to HCPs. Jones, Parnell, and Hardaway owned
numerous MSOs, including Alpha Rise Health LLC (Alpha Rise), Beta Rise Health LLC (Beta
Rise), and Omega Rise Health LLC (Omega Rise) (collectively, “Rise MSOs”). Jones, Parnell,
and Hardaway used the Rise MSOs to kickback thousands of dollars to HCPs who referred
laboratory testing and other diagnostic services to LRH.
235. The below chart summarizes over $1 million in Rise MSO payments to referring
HCPs to induce their referrals to LRH:
HCP
MSO
MSO Payments
Aria Dayani and Divya Muthappa Alpha Rise; Beta Rise $96,634.72
Aria Dayani and Saleh Jaafar Alpha Rise $46,396.48
Dagberto Balderas
Alpha Rise
$27,649.00
Dan Freeland Alpha Rise $36,096.48
David Sneed Alpha Rise $161,185.92
Edward Miwa
Alpha Rise
$46,396.48
Gary Goff Alpha Rise $46,396.48
John Hierholzer Alpha Rise $12,425.00
Jose Ortiz Alpha Rise $40,296.48
Ken Locke
Alpha Rise
$30,638.48
Kevin Lewis Alpha Rise $28,662.00
Marc Krock Omega Rise $8,115.86
Marco Munoz
Alpha Rise
$46,396.48
Maricela Mazuca Omega Rise $9,700.00
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 57 of 154 PageID #: 940

58
HCP MSO MSO Payments
Matthew Thompson Alpha Rise $44,496.48
Melissa Miskell
Alpha Rise
$50,196.48
Mitch Finnie Alpha Rise $66,959.96
Patricia Allen Omega Rise $12,815.86
Rae Benson
Omega Rise
$11,815.86
Raymon Garcia Alpha Rise $46,396.48
Saleh Jaafar Beta Rise $81,123.00
Stephanie Berg and Andrew Minigutti Alpha Rise $46,396.48
Tad Titlow
Omega Rise
$44,926.88
236. To disguise the kickbacks, Jones, Parnell, and Hardaway used purported
“investment” documentation for the Rise MSOs. Only HCPs who referred to LRH were allowed
to participate and remain in the Rise MSOs, and the only source of revenue for the Rise MSOs
came from the referrals or other business generated by the HCPs in the MSOs. While HCPs
purported to invest in the Rise MSOs, the MSOs’ payments to HCPs were not based on the returns
from any genuine investment. Instead, the Rise MSOs’ payments to HCPs were simply profits
shared with HCPs based on the HCPs’ referrals to LRH.
237. In or about June 2015, Jones provided Madison with “the MSO handout we will be
providing physicians,” which included a detailed explanation of Rise MSOs’ sales pitch to HCPs.
The Rise MSO sales pitch was explicitly offered to only “Health Care Provider[s]” and offered
them “investment opportunities” at “$1000.00 per share with a limit of two shares.” In the Rise
MSO sales pitch, Jones indicated that HCPs could order laboratory services contracted through
LRH, including BHD blood tests, Asperio Labs genetic and toxicology testing, and Essential Labs
toxicology testing. Jones provided Madison with Rise MSOs’ “sales forecast,” projecting that,
based on referrals from HCPs in the MSO, LRH would receive by August 2015 reimbursements
of $102,000 from BHD testing, $25,500 from genetic testing, and $6,800 from toxicology testing,
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 58 of 154 PageID #: 941
59
with LRH’s reimbursements rising as more HCPs joined Rise MSO, and totaling an estimated
$11.3 million in the first year. Jones also provided Madison with Rise MSOs’ “pro forma cash
flow,” projecting that the “physician divident [sic] payment – 1 share” would be $997 in the first
month, $3,472 in the second month, and a total of $88,483 in the first year.
238. According to the Rise MSO pro forma, an HCP who “invested” $1,000 for one
share in a Rise MSO would recover almost her entire investment amount in the first month and
receive an 8,748% return on investment in the first year. Rise MSOs’ purported dividend payments
to HCPs were not based on any genuine investment, but were a share of the profits generated by
the HCPs’ referrals to LRH.
239. Madison understood and agreed to Rise MSOs’ plan to pay HCPs to induce them
to order laboratory testing and other diagnostic services from LRH.
240. Jones, Parnell, and Hardaway provided to HCPs the same Rise MSO
documentation that Jones had provided to Madison. In communications with HCPs, they explained
and elaborated on the MSO kickback scheme. As Jones noted in his pitch to an HCP in or about
June 2015, “[w]e are an MSO that has contracted with [LRH]” and the “advanced testing we
represent are [BHD] advanced lipid testing” as well as “cutting edge Genetic Testing and
toxicology.” The Rise MSO pitch emphasized how much money HCPs could earn from their
referrals, offering HCPs “the opportunity to become owners in the MSO and receive profit sharing
based on the performance of the MSO and the amount of share ownership.”
241. At the direction of Madison and Borgfeld, from in or about September 2015 through
at least May 2016, LRH paid at least $3,197,054 to LGRB for arranging for and recommending
referrals to LRH and to fund the MSO kickbacks to referring HCPs. LGRB, in turn, transferred
the funds during that period to the Rise MSOs, with Alpha Rise receiving at least $2,663,690, Beta
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 59 of 154 PageID #: 942
60
Rise receiving at least $318,125, and Omega Rise receiving at least $215,240. During that period,
the Rise MSOs kicked back over $1 million of those funds to the referring HCPs.
242. For example, in or about July 2015, LGRB recruited Gary Goff, M.D. (Physician
F), of Dallas, Texas, to refer to LRH for laboratory testing. To induce Physician F’s referrals to
LRH, LGRB offered to pay MSO kickbacks to Physician F through Alpha Rise. Physician F agreed
to participate in the kickback scheme and began referring laboratory tests, including for federal
healthcare beneficiaries, to LRH in or about August 2015.
243. Before LGRB offered to pay MSO kickbacks to Physician F through Alpha Rise,
Physician F had never previously ordered laboratory tests from LRH. Physician F did not have
admitting privileges at LRH. Physician F had never practiced at LRH. And Physician F had never
referred any patient to LRH before agreeing to participate in the MSO kickback scheme. Indeed,
Physician F’s medical practice in Dallas, Texas was over 160 miles away from LRH’s Rockdale,
Texas facility.
244. Alpha Rise paid Physician F over $46,000 in MSO kickbacks, a return of 2,200%
based on Physician F’s purported investment of $2,000. In addition to paying Physician F the MSO
distributions, Alpha Rise returned to Physician F his full purported investment amount in 2017.
245. From in or about August to December 2015, during his participation in LGRB’s
kickback scheme, Physician F referred to LRH hundreds of tests payable by federal healthcare
programs. LRH submitted those claims to federal healthcare programs as purported outpatient
services, and Medicare paid over $100,000 to LRH. Examples of those claims are included in
Exhibit A hereto.
246. For their role in the LRH kickback scheme, Jones, Parnell, and Hardaway received
over $1 million, including management fees and MSO payments.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 60 of 154 PageID #: 943
61
E. LRH and the Recruiters Partnered with Two Laboratory Co-Conspirators
247. For their laboratory fraud scheme to succeed, Madison and Borgfeld knew they
would need to partner with a clinical laboratory to run the tests ordered by the HCPs. LRH did not
have the capability in 2015 to perform specialized laboratory testing, lacking the needed personnel
and laboratory equipment, among other things. LRH first partnered with BHD, and later with
BHD’s competitor, THD.
248. For a fee, both BHD and THD allowed LRH to bill their blood tests to insurers,
including federal healthcare insurers, as purported hospital outpatient services. By billing for
purported outpatient services, LRH charged insurers a much higher rate than BHD or THD could
receive as clinical laboratories or that LRH could receive for laboratory tests for hospital non-
patients.
249. When Theiler discussed the LRH arrangement with Hertzberg in December 2015,
he noted that LRH “[q]ualifies as a ‘Critical Access Hospital’ which is a hospital certified under a
set of Medicare conditions,” including “[b]eing located in a rural area, at least 35 miles drive away
from any other hospital.” Theiler noted to Hertzberg that LRH’s CAH status allowed it to “receive
cost-based reimbursement from Medicare, instead of standard fixed reimbursement rates.”
250. That month, Hertzberg highlighted to colleagues the significant reimbursement
increase when LRH submitted the claims, stating that hospitals like LRH “are paid a premium (3x
standard rate) by government and private payors.” As Theiler understood, “[w]ith the favorable
reimbursement, [LRH] will assume responsibility of billing insurance” for the laboratory tests.
251. Likewise, in internal documents approved by Grottenthaler in 2016, THD described
its “unique hospital strategy” and “hospital partnership model” in which “[THD’s] tests are white-
labeled and marketed as the hospital’s tests.” THD’s documents highlighted the “increased
economic opportunity accessed via unique operating partnerships,” noting that typical
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 61 of 154 PageID #: 944
62
reimbursement when THD bills the laboratory tests is about “$300 – $315,” whereas the
reimbursement when a hospital bills the laboratory tests is much higher, such that THD’s share of
the hospital’s reimbursement is “$500+” and even “$600 – $650 per sample for existing hospital
partnerships,” including LRH.
252. At Grottenthaler’s direction, THD focused heavily on the hospital strategy, aiming
for “rapid growth (2016 and beyond)” based on an “accelerated roll out” of “highly accretive
hospital partnership agreements.” By having LRH and other hospitals bill its tests, THD sought to
“increas[e] revenue from hospitals,” which “will more than offset projected decline in
reimbursement rates from Medicare and commercial insurers” under the CLFS.
253. Hertzberg, Theiler, and Howard knew that the individuals receiving BHD testing
through LRH were neither inpatients nor outpatients of LRH because BHD personnel participated
with MSO recruiters in sales visits to the referring HCPs and understood that the individuals were
patients of the HCPs, not hospital patients. For the same reason, Grottenthaler, Kash, and Love
knew that the individuals receiving THD testing through LRH were not LRH patients.
254. BHD and THD executives and sales personnel knew that LRH billing their
laboratory tests as purported services to hospital patients would result in false, inflated claims to
insurers, including federal healthcare insurers, and higher reimbursements for their labs.
255. To further the false claims conspiracy, both BHD and THD identified HCP targets
for the LRH-affiliated MSOs, referred HCPs interested in kickback payments to the MSOs to
secure their blood testing referrals, and participated with the MSOs in sales pitches to offer HCPs
money to induce their referrals.
1. BHD Executives and Sales Personnel Joined the Conspiracy
256. On or about December 5, 2014, Hertzberg signed a merger agreement to transfer
100% of the shareholding in BHD to Eurofins Clinical Testing US Holdings, Inc. (Eurofins). The
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 62 of 154 PageID #: 945
63
merger closed on or about January 30, 2015. Pursuant to the merger agreement, the purchase price
consisted of a closing payment plus a contingent “earnout” payment. The earnout payment was to
be calculated based on BHD’s profitability during 2016 and 2017 (earnout period). As part of the
earnout provisions, Hertzberg, Theiler, and other BHD executives would remain in place with
significant managerial independence from Eurofins during the earnout period. Hertzberg and
Theiler stood to receive about 7.9 percent and 1.3 percent, respectively, of the earnout payment,
depending on BHD’s profitability during the earnout period.
257. Shortly after the merger closed, a physician who had a financial relationship with
LRH alerted Hertzberg that LRH’s CEO, Madison, was reaching out to a competitor laboratory to
discuss a potential “lucrative deal.” The physician told Hertzberg that LRH “want[s] to bill for
labs themselves” because they have “great” contracts with payors. Hertzberg replied, “I’m on it!
Stay tuned!” The physician then gave Madison’s contact information to Hertzberg.
258. On or about April 1, 2015, Hertzberg approved LRH’s proposed arrangement with
BHD, and Madison signed the agreement. Described as a “buy and bill contract,” Hertzberg
allowed LRH to bill BHD tests to insurers, including federal healthcare programs, in return for a
fee paid to BHD.
259. In or about April 2015, LRH performed a “test pilot” of submitting one physician’s
BHD tests to insurers as purported hospital outpatient laboratory testing. Once they saw that the
billing scheme generated significantly more reimbursement, based on a CAH submitting the claims
rather than a clinical laboratory, Madison and Borgfeld began paying MSO recruiters to arrange
for or recommend that HCPs order BHD testing through LRH.
260. BHD’s sales force, with Hertzberg and Theiler’s knowledge and approval, worked
closely with LRH and the recruiters who paid MSO kickbacks to induce referrals to LRH for BHD
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 63 of 154 PageID #: 946
64
testing. Hertzberg spoke with Madison about the LRH arrangement on numerous occasions.
Parnell, Hardaway, Howard, and other BHD personnel identified HCP targets for the LRH-
affiliated MSOs, referred HCPs interested in kickback payments to the MSOs to secure their blood
testing referrals, and participated with the MSOs in sales pitches to offer HCPs money to induce
their referrals.
261. For example, in or about May 2015, a BHD Regional Sales Director who reported
to Theiler confirmed to Parnell and Hardaway that he had joined MSO recruiters at a dinner to
recruit six physicians to order BHD tests through LRH; after the MSO pitch, “4 [physicians] have
moved forward with joining the MSO.” He highlighted the impact of the MSO pitch on a physician
who had referred a large volume of tests to another laboratory and had planned to meet with a
competitor: “After [the MSO recruiters] discussed the MSO, he is probably going to use us.”
262. Fueled by the MSO kickbacks, referrals to LRH for BHD testing increased rapidly.
One LRH-affiliated physician told Hertzberg in or about June 2015, “We’ve been smoking it!
Hundreds and hundreds of labs. Gray [Hardaway] is beside himself!”
263. As the LRH arrangement progressed, Hertzberg and Theiler closely tracked the
revenue that BHD received from the arrangement. In or about July 2015, Hertzberg and Theiler
reviewed data showing that “[LRH] growth continues to be very strong,” with a BHD employee
advising Hertzberg that if they “annualize the [LRH] volume,” BHD would be “up a net of $4.4M.”
Hertzberg replied, “Have we gotten paid???” A BHD employee confirmed to Hertzberg, “YES!!
$300k came in at the end of last week – they are going to be wiring us weekly.”
264. Hertzberg and Theiler continued to track the financial success of the LRH
arrangement. In or about September 2015, Hertzberg and Theiler reviewed data showing that BHD
had received over $1 million to date from LRH. Based on the average LRH orders for BHD testing
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 64 of 154 PageID #: 947
65
over the past four weeks, BHD’s annualized revenue related to LRH would be $20,866,560—an
increase of $19,166,560 from BHD’s “base business” without LRH. Upon reviewing those revenue
numbers and a graph of the steeply rising rate of LRH referrals, Hertzberg contacted Theiler and
other BHD executives, exclaiming “WOWIE!!!!! HOW DO WE GET SOME MORE OF
THAT???!!!!!!!”
265. The next month, a BHD sales director informed Hertzberg that Grottenthaler—the
CEO of THD, a competitor laboratory to BHD—had spoken with Madison, and that “[THD] is in
negotiations with [LRH] for a similar model/arrangement as BHD.” Hertzberg replied that “I
would expect everyone is talking to them [LRH].” To preserve BHD’s revenue from the LRH
arrangement, Hertzberg told a BHD sales director: “[W]e need to keep our touch high and service
levels even higher!”
266. The sales director agreed but noted to Hertzberg that BHD had “problems” in
“filtering clients with pure intent” who were referred by an MSO working with LRH. The sales
director also warned Hertzberg about BHD’s experience working with an LRH-affiliated “MSO
called Benchmark,” which was “misleading and dirty.” The sales director told Hertzberg that THD
was “associat[ed] with unethical/underhanded practices and people” and “Benchmark is going to
[THD] with some of its referrals.”
267. Despite the problems, Hertzberg and Theiler continued the lucrative LRH fraud
scheme, without disclosing the problems or the MSO kickbacks to BHD’s parent company.
268. Given the substantial revenue the LRH arrangement was generating for BHD,
Hertzberg worked with Theiler on plans to expand the arrangement into a formal joint venture, to
prevent LRH from working with BHD’s competitor, THD. Under Hertzberg and Theiler’s
proposed joint venture, BHD would have helped LRH develop and operate an on-site laboratory.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 65 of 154 PageID #: 948
66
269. Hertzberg and Theiler knew why the existing LRH arrangement was lucrative. As
a BHD sales director highlighted to Theiler in or about October 2015, the MSOs “work with
[LRH],” “practitioners partner[] with MSO” for their testing and “share in profits of MSO,” and
BHD receives leads from the MSOs for new HCP clients. The sales director noted to Theiler that
MSOs offer the “testing, both [BHD] and toxicology, through [LRH].” Despite calling themselves
MSOs, the BHD sales director noted to Theiler that “the MSOs working with [LRH] are not
providing any management/administrative service for the office.”
270. The joint venture contemplated by Hertzberg and Theiler to develop LRH’s on-site
lab would have required approval by BHD’s parent company in Europe. At Theiler’s request, a
BHD sales director, with assistance from Parnell and Hardaway, prepared an executive summary
in or about December 2015 of the existing LRH arrangement for Hertzberg to use when discussing
her proposed LRH joint venture with BHD’s parent company. The summary explained that LRH’s
“unique” status as a CAH gave it “very favorable reimbursement,” allowing it to “receive cost-
based reimbursement from Medicare, instead of standard fixed reimbursement rates.” BHD’s
summary acknowledged that cost-based reimbursement was designed “to enhance the financial
performance of small rural hospitals” like LRH. The summary noted that LRH had “10 patient
beds” and “originally served the Central Texas area, but over the last year, has increased [its]
relationships with medical providers in Houston, Dallas and other cities in Texas and Oklahoma.”
271. The executive summary prepared at Theiler’s request also described the kickbacks
that LRH used to induce HCPs to refer laboratory testing to LRH, explaining that a “driver for
growth for [LRH] and other hospitals is the [MSO] model.” The summary noted that “[LRH] will
employ Marketers. These Marketers represent [MSOs]. The practitioners to [sic] become investors
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 66 of 154 PageID #: 949
67
by purchasing shares in the MSOs. [LRH] will remunerate the Marketers/MSO, which in turn
disperse their profits among the investors.”
272. Hertzberg and Theiler knew of the broad reach of LRH’s MSOs in recruiting HCPs
to order BHD tests through LRH. In or about October 2015, Theiler noted to Hertzberg that “the
MSO [for LRH was] recruiting physicians outside of Austin and into other markets.” BHD’s VP
for Hospital Strategy confirmed this point to Hertzberg and Theiler a few days later, noting that
LRH’s marketers were recruiting “way outside of the [LRH] access area for patients,” even though
a “[CAH] exists to provide access and does not typically have a marketing arm.” The VP gave
Hertzberg and Theiler her “strong recommendation and request” to “reel this in” and “stand down
on all hospitals, particularly in [Texas].”
273. Hertzberg and Theiler chose not to end BHD’s participation in the MSO kickback
scheme, aware of the lucrative nature of their LRH arrangement. Instead, Hertzberg and Theiler
tracked the “LR[H] accounts, with volumes, how they were put under LR[H], and how they were
in-serviced [by BHD].” In or about November 2015, Theiler sent Hertzberg a detailed spreadsheet
listing, among other things, the names, referral volumes, and referral start dates for 128 HCPs for
whom an MSO relationship was the “source of referral to [BHD],” who were listed as responsible
for 2,185 referrals in just the past month. Theiler even forwarded to Hertzberg the name and phone
number of “MSO/Marketer Marty Flores,” who was a recruiter for the Next Level MSOs.
274. Despite discussing the proposed LRH joint venture with BHD’s parent company,
Hertzberg and Theiler did not tell that company about the MSO kickbacks.
275. BHD’s parent company did not approve Hertzberg’s proposed joint venture to
develop LRH’s on-site lab, but that decision did not dissuade Hertzberg and Theiler. They
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 67 of 154 PageID #: 950
68
continued BHD’s preexisting arrangement with LRH, in which BHD performed the laboratory
testing, the recruiters paid the MSO kickbacks, and LRH submitted claims to insurers for the tests.
276. As intended by Hertzberg and Theiler, BHD’s participation with LRH and others
in the hospital billing and MSO scheme was highly lucrative, with LRH paying BHD over $30
million from in or about July 2015 to February 2018.
2. THD Executives and Sales Personnel Joined the Conspiracy
277. By about August 2015, Grottenthaler, Cornwell, and Love realized that THD was
losing business in Texas because certain HCPs were ordering BHD tests to receive money from
MSOs.
278. Initially, Grottenthaler tried to launch “a pilot-project” for what Cornwell described
(in an email copying Grottenthaler) as “our MSO,” referring to an MSO based in Dallas, Texas.
279. Given the importance of the MSO strategy to THD, it was Grottenthaler who
“helped us [THD] set this up” with the MSO. As Grottenthaler, Cornwell, and Love understood,
the MSO would pay HCPs for laboratory referrals, THD would perform the laboratory testing for
the MSO for a fee, and the MSO would bill the claims to commercial and federal insurers.
280. Grottenthaler helped set up and approved the plan, and Cornwell helped to
implement it. However, when the MSO tried to bill insurers for THD’s laboratory tests, it faced
difficulties, unlike established hospitals, in being paid by insurers.
281. Aware that he needed hospitals for the scheme to work, Grottenthaler hired
defendant Kash in October 2015. Kash had a close relationship with O’Neal, who operated the
Ascend MSO with Hickman, and O’Neal had a close relationship with a Texas hospital executive,
LRH’s CEO Madison.
282. Grottenthaler knew that THD’s competitor, BHD, already had partnered with LRH,
using MSOs to gain business at THD’s expense. Kash advised Grottenthaler that partnering with
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 68 of 154 PageID #: 951
69
LRH would “allow us to get another competitive response, to address what is going on here in
Texas.”
283. Grottenthaler, Kash, O’Neal, and Madison had numerous meetings in person and
by phone and ultimately agreed that THD would join the LRH scheme.
284. On or about November 2, 2015, LRH entered into a laboratory processing
agreement with THD. Pursuant to the agreement, LRH agreed to pay THD for performing
laboratory tests on specimens that LRH sent to THD, and THD allowed LRH to bill the tests to
any public or private insurer. Borgfeld signed the agreement for LRH, and Grottenthaler signed
for THD.
285. Grottenthaler sought to encourage the hospital referrals by, among other things,
paying commissions to THD’s sales force for tests ordered from a hospital if the tests had been
performed by THD.
286. Cornwell, Kash, and Love welcomed Grottenthaler’s decision to join the LRH
kickback scheme. They believed that the MSO payments would increase THD’s business,
including business lost to BHD. As Cornwell told Grottenthaler, having Kash and Love coordinate
with Ascend MSO, for example, would be “an opportunity to get [a high-volume HCP account’s]
business back” after the HCP had switched to “sending over 100 BH[D] tests per week to LR[H]
through Ascend’s MSO[.]” Numerous high-volume HCP accounts had been “lost to [BHD’s] MSO
(presumably Dr. O’Neill’s [sic]),” Love told Cornwell. After learning of THD’s arrangement with
LRH, Love said “it is good to have a competitive response to [BHD’s] strategy,” even though she
had “a big concern [about] what happens to these accounts once they move over to a MSO,” in
light of all of “the MSO’s marching around town.”
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 69 of 154 PageID #: 952
70
287. Kash, Cornwell, and THD’s employed sales representatives, including Love, spoke
to HCPs about the MSO kickbacks, referred HCPs to MSO recruiters, and referred MSO recruiters
to HCPs. The MSO scheme became a key part of THD’s business, with Love telling Cornwell that
“[o]ur business hinges on how well the hospitals and MSOs work together.”
288. Given the lucrative nature of the LRH arrangement, Grottenthaler sought to deepen
THD’s relationship with LRH. On or about May 1, 2016 and December 19, 2016, THD-Outreach
agreed with LRH to develop and manage a laboratory onsite at LRH and to prohibit LRH from
receiving such services from any competitors to THD (such as BHD). Pursuant to both agreements,
LRH agreed to pay THD per laboratory test that was performed, and THD allowed LRH to bill the
tests to any public or private insurer. Grottenthaler signed both agreements, and Borgfeld signed
the December 2016 agreement.
289. LRH and THD-Outreach also entered into an equipment lease agreement on or
about December 21, 2016, signed by Grottenthaler and Borgfeld, and a consulting agreement on
or about February 15, 2017, also signed by Grottenthaler.
290. On or about April 1, 2017, THD affiliate THD-Financial agreed to submit
laboratory claims in LRH’s name and under LRH’s National Provider Identifier (NPI) to insurers,
including federal healthcare programs, in return for LRH paying 7% of net collections to THD-
Financial.
291. As intended by Grottenthaler, THD and its affiliates’ participation with LRH and
others in the hospital billing and MSO scheme was highly lucrative, with LRH paying THD, THD-
Outreach, and THD-Financial over $15.9 million.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 70 of 154 PageID #: 953

71
F. LRH Submitted False Claims to Medicare, Medicaid, and TRICARE
292. During the period of January 1, 2015 to December 31, 2017, LRH submitted false
claims for laboratory testing to federal healthcare programs. Example claims are identified in
Exhibit A hereto.
293. As LRH’s final claims for payment for Medicare services, including toxicology and
blood testing, for the below time periods, LRH submitted to CMS its annual cost report on Form
CMS-2552 on the below dates.
Cost Report Period Date Submitted LRH Signatory Date Signed
1/1/2014 – 12/31/2014 6/1/2015 Borgfeld 5/28/2015
1/1/2015 – 12/31/2015 6/1/2016 LRH’s CFO 5/31/2016
1/1/2016 – 12/31/2016 6/1/2017 Madison 5/31/2017
1/1/2017 – 12/31/2017 6/1/2018 Borgfeld 5/31/2018
294. Each of LRH’s cost reports for years 2014 through 2017 included the certifications
in paragraphs 42–46 above, signed by the above LRH signatories.
II. OTHER TEXAS HOSPITALS
295. In light of the success of the LRH kickback scheme, a number of its co-conspirators
agreed to implement the MSO kickback scheme with other Texas hospitals. To induce HCPs’
referrals for diagnostic services reimbursed by federal healthcare programs, including laboratory
tests, the co-conspirators agreed to a scheme to pay thousands of dollars to referring HCPs, while
disguising the payments as purported MSO investment distributions.
A. Integrity Transitional Hospital Fraud Scheme
296. In late 2015, BHD and THD joined another MSO kickback scheme involving a
Texas hospital. The hospital was a long term care hospital (LTCH) in Denton, Texas named
Denton Transitional LTCH, L.P. d/b/a Integrity Transitional Hospital (ITH).
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 71 of 154 PageID #: 954
72
297. An LTCH is a hospital that, among other things, “is primarily engaged in providing
inpatient services, by or under the supervision of a physician, to Medicare beneficiaries whose
medically complex conditions require a long hospital stay and programs of care provided by a
long-term care hospital.” 42 U.S.C. § 1395x(ccc). With limited exceptions, an LTCH “has an
average inpatient length of stay . . . of greater than 25 days.” Id.
298. Rather than focusing on performing laboratory tests for ITH’s long-term inpatients,
BHD and THD sought to partner with ITH as part of an MSO kickback scheme to induce HCPs to
refer laboratory testing for non-patients of ITH.
299. In or about July 2015, Hardaway introduced the ITH arrangement to BHD, noting
that his contact at ITH “know[s] the [LRH] folks.” Hardaway’s contact at ITH was Benchmark
Medical LLC (Benchmark), one of ITH’s MSO recruiters, which had worked with BHD in the
LRH scheme.
300. Aware of the lucrative nature of the LRH arrangement, Hertzberg and Theiler
approved entering into a similar arrangement with ITH.
301. Effective on or about August 10, 2015, BHD entered into a laboratory services
agreement with ITH. Pursuant to the agreement, ITH agreed to pay BHD for performing laboratory
tests on specimens that ITH sent to BHD.
302. For patients covered by commercial insurance, ITH billed commercial insurers and
agreed to pay a per-test fee to the laboratory that performed the testing. For patients covered by
Medicare, Medicaid, and TRICARE, BHD billed the federal healthcare programs.
303. Hertzberg and Theiler sought to use ITH to implement the LRH fraud schemes at a
new hospital. Hertzberg and Theiler knew that the MSO kickbacks that LRH’s recruiters had paid
to induce referrals would be replicated at ITH. A BHD sales director informed Hertzberg and
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 72 of 154 PageID #: 955
73
Theiler that “[ITH] as a lead was derived from a group of marketers called Benchmark. Benchmark
was originally with [LRH] but [LRH] cut ties with them.” The sales director had previously told
Hertzberg that LRH cut ties with an “MSO called Benchmark” because Benchmark was
“misleading and dirty.”
304. To implement the ITH arrangement, as Theiler and Hertzberg knew, “Gray
[Hardaway] and Jeff [Parnell] work completely through the CEO of Benchmark (Eric) in their
dealings with [ITH].” BHD’s sales force, including Hardaway and Parnell, identified HCP targets
for the ITH-affiliated MSOs, including Benchmark, referred HCPs interested in kickback
payments to the MSOs to secure their blood testing referrals, and participated with the MSOs in
sales pitches to offer HCPs money to induce their referrals.
305. As LRH had done, ITH invited THD to join the ITH arrangement in or about
September 2015. Love spoke with an ITH executive about the proposed arrangement, and she,
Grottenthaler, and Cornwell then met with two ITH executives, including its CEO, in or about
October 2015.
306. After the ITH meeting, Grottenthaler and Love met with Paul Worrell, D.O.
(Physician G), of Dallas, Texas, to try to gain his business.
307. Shortly after the Physician G meeting, Love, Grottenthaler, Cornwell, and an ITH
executive communicated in or about October 2015 about the financial inducements needed to gain
laboratory referrals from HCPs, including Physician G. Love explained to Grottenthaler and
Cornwell that Physician G “has a large volume clinic that averages around 80 specimens per week
that is predominantly 3rd party.”
308. In or about October 2015, Love gave ITH the Rise MSO handout referenced above
in paragraphs 237–238, which Rise had provided to Physician G, telling him that he could “buy
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 73 of 154 PageID #: 956
74
additional shares based on his volume[.]” ITH responded to Love, in an email shared with
Grottenthaler and Cornwell, indicating that “the best option for the physician will be a direct
investment into a lab clia” to allow the physician to “own a clean 40%.” ITH indicated that the
physician-owned laboratory structure is “less scrutinized than MSOs and there is no payment that
has to be made up to the management company of the MSO for them getting involved in the
practice.” ITH requested “the list of CPT codes that [THD] processes in their panel because the
only way I can figure out what we will charge/make from the blood tests is if I have those codes.
That way I can get a pro forma made.” The pro forma would show HCPs, including Physician G,
how much money they would receive from their referrals as part of the kickback scheme.
309. To assist ITH in preparing a pro forma, Love asked Grottenthaler whether THD’s
CFO was “the best resource for CPT codes.” THD’s CFO provided the CPT codes for THD’s tests,
and Love provided those codes to ITH in or about October 2015.
310. After receiving the Rise MSO handout from Love, ITH forwarded it to Darious
Shafie on or about October 19, 2015.
311. About a week later, on or about October 26, 2015, Shafie formed BDS Healthcare,
LLC, d/b/a Vybrem Labs (Vybrem), a Texas company he owned and operated. Shafie formed
Vybrem to pay kickbacks to HCPs for laboratory referrals. Shafie created a Vybrem MSO handout
nearly identical to the Rise MSO handout that he had received from Love.
312. On or about October 26, 2015, Love, Cornwell, Shafie, and an ITH executive met
with Physician G and offered MSO kickbacks to Physician G to induce him to order THD
laboratory tests as part of the ITH scheme. Physician G agreed to participate in the kickback
scheme with THD and ITH.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 74 of 154 PageID #: 957
75
313. Effective on or about November 1, 2015, THD entered into a laboratory services
agreement with ITH, signed by Grottenthaler and ITH’s CEO. Pursuant to the agreement, ITH
agreed to pay THD for performing laboratory tests on specimens that ITH sent to THD.
314. Although Grottenthaler had offered to pay THD’s sales force a “$50k bonus for any
hospital that implements and pays for its first 500 samples,” Grottenthaler refused to pay such
bonus for ITH because he previously had been “introduced to [ITH]” through another source.
315. For patients covered by commercial insurance, ITH billed commercial insurers and
agreed to pay a per-test fee to the laboratory that performed the testing. For patients covered by
Medicare, Medicaid, and TRICARE, THD billed the federal healthcare programs.
316. To recruit HCPs to order the laboratory testing, ITH paid commissions to MSO
recruiters. ITH’s MSO recruiters then kicked back a portion of the commissions to referring HCPs,
disguising the remuneration as MSO distribution payments when in fact it was paid to induce the
HCPs’ commercial and Medicare, Medicaid, and TRICARE referrals to ITH, THD, and BHD for
laboratory testing.
317. For example, from in or about November 2015 to June 2016, during his
participation in the Vybrem MSO kickback scheme, Physician G referred to THD hundreds of
tests payable by federal healthcare programs. THD submitted those claims to federal programs,
including Medicare, and examples of those claims are included in Exhibit A hereto.
318. As agreed with THD and BHD, ITH paid the phlebotomists and other medical staff
in HCPs’ offices to draw the blood for patients with federal healthcare insurance and patients with
commercial insurance. ITH, ITH’s recruiters, THD, and BHD instructed the phlebotomists to
collect insurance information for federal healthcare beneficiaries and provide that information to
THD or BHD, so that THD or BHD could bill for the claims.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 75 of 154 PageID #: 958

76
319. To fund the MSO kickbacks, ITH paid commissions to numerous MSO recruiters,
including Vybrem. During the period of in or about October 2015 to June 2016, ITH paid Vybrem
over $450,000.
320. The below chart summarizes over $200,000 in Vybrem MSO payments from in or
about October 2015 to June 2016 to referring HCPs for their laboratory referrals:
HCP MSO Payments
Paul Worrell $67,044
Jason Finkelstein
$29,322
Charles Evans $58,653
Joseph Scott and Brent Gorman $46,121
321. To disguise the kickbacks, Shafie used purported “investment” documentation for
the Vybrem MSO. Only HCPs who referred diagnostic services, including laboratory testing, as
part of the ITH scheme were allowed to participate and remain in the Vybrem MSO, and the only
source of revenue for the Vybrem MSO came from the referrals or other business generated by the
HCPs in the MSO. While HCPs purported to invest in the Vybrem MSO, the MSO’s payments to
HCPs were not based on the returns from any genuine investment. Instead, the Vybrem MSO’s
payments to HCPs were simply profits shared with HCPs based on the HCPs’ referrals as part of
the ITH scheme.
322. Aware that he was being paid for referrals, Charles Evans, M.D., of Lufkin, Texas,
asked Shafie in or about January 2016 about “other tests that I might run through Vybrid [sic]”
because “[t]he more we add to the list the more we make[.]” Evans told Shafie that when Cornwell
visits Evans’ office, Cornwell should “please make sure he presents this as an arrangement
between [THD] and [ITH]. My name and Vybrid [sic] labs (our association) should never come
into the conversation.” Evans noted, “[m]y entire staff know him [Cornwell] well and some are
aware that he has presented me with a business proposition with Vybrid [sic] labs.” Evans
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 76 of 154 PageID #: 959
77
acknowledged that he had told his staff the admittedly “misleading” answer that “I am not
interested in participating with any MSO scams, and our focus has to remain on our patients, not
our bank books!”
323. As intended by Grottenthaler and Hertzberg, THD’s and BHD’s participation with
ITH and others in the MSO scheme was lucrative, with ITH paying THD over $1 million in 2016
and BHD over $5.1 million that year.
B. Stamford Memorial Hospital Fraud Scheme
324. From late 2015 to early 2016, Grottenthaler, Kash, and O’Neal solicited another
rural hospital in Texas to participate in their laboratory fraud scheme. They targeted a small
hospital with 25 or fewer beds named Jones County Regional Healthcare d/b/a Stamford Memorial
Hospital (Stamford) in Stamford, Texas (population under 4,000).
325. Kash and O’Neal met with Stamford’s CEO on or about December 29, 2015 to
discuss a laboratory billing arrangement based on the LRH model. O’Neal’s partner subsequently
provided Stamford’s CEO with “actual figures” from LRH, pointing to the “remarkable” and
“explosive growth” generated by the hospital billing for blood tests, noting that the associated
revenue to the hospital was over $94 million and “entirely incremental for the Hospital.”
326. Grottenthaler and Kash met with O’Neal on multiple occasions, including on or
about March 7, 2016 in Frisco, Texas, and discussed the potential laboratory arrangement with
Stamford. To secure Stamford’s participation, Grottenthaler and O’Neal met with Stamford’s CEO
and had numerous communications with him, including an in-person meeting on or about March
22, 2016.
327. Effective on or about May 6, 2016, THD entered into a laboratory processing
agreement with Stamford. Pursuant to the agreement, signed by Grottenthaler and Stamford’s
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 77 of 154 PageID #: 960
78
CEO, Stamford agreed to pay THD for performing laboratory tests on specimens that Stamford
sent to THD.
328. Shortly thereafter, effective on or about May 23, 2016, THD-Financial entered into
a laboratory billing and collection agreement with Stamford, signed by Grottenthaler and
Stamford’s CEO. Pursuant to the agreement, THD agreed to provide billing and collection services
to Stamford “in connection with [Stamford] Hospital’s expanded laboratory program,” including
“to submit, in the name of [Stamford] Hospital and any and all laboratory related Professional
Services for the benefit of [Stamford] Hospital under [Stamford] Hospital’s provider number(s),
and on [Stamford] Hospital’s behalf, all claims for reimbursement to all patients and third party
payors, including, without limitation, state or federal health care programs, for all Professional
Services provided by [Stamford] Hospital to patients”; and “to collect and receive, in the name of
[Stamford] Hospital, and on behalf of [Stamford] Hospital, all accounts receivable generated by
claims for reimbursement.” Stamford agreed to pay THD-Financial 5 percent of net collections for
billing and collecting the laboratory claims.
329. During the same time period, on or about January 12, 2016, O’Neal met with BHD
representatives Theiler and Howard in Dallas, Texas.
330. On or about February 11, 2016, O’Neal had a conference call with Theiler and a
BHD representative. Theiler took notes and emailed the notes to a BHD representative.
331. On or about March 4, 2016, Theiler met with O’Neal in Beaumont, Texas.
332. In or about April 2016, Theiler decided to join the Stamford arrangement. Between
April and June 2016, Theiler had multiple communications with O’Neal and Stamford’s CEO and
discussed the arrangement and negotiated contract pricing with them.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 78 of 154 PageID #: 961
79
333. Effective on or about May 31, 2016, BHD entered into a laboratory services
agreement with Stamford. Pursuant to the agreement, which Theiler negotiated and sought
approval for within BHD, Stamford agreed to pay BHD for performing laboratory tests on
specimens that Stamford sent to BHD. Stamford’s CEO emailed the signed agreement to Theiler.
334. Under their arrangements with Stamford, THD and BHD agreed to perform
laboratory testing for Stamford for a per-test fee.
335. As part of the Stamford arrangement, for laboratory tests THD performed from on
or about May 2016 to December 2016, THD-Financial billed commercial insurers using
Stamford’s name and NPI, and THD billed federal insurers using THD’s and NPI. Thereafter, once
Stamford and THD-Financial terminated their billing and collection agreement effective on or
about December 13, 2016, Stamford billed commercial insurers using Stamford’s name and NPI,
and THD billed federal insurers using THD’s name and NPI.
336. As part of the Stamford arrangement, for laboratory tests BHD performed,
Stamford and/or its contractor billed commercial insurers in Stamford’s name using Stamford’s
NPI, and BHD billed federal insurers in BHD’s name using BHD’s NPI.
337. In coordination with the laboratories and recruiters, Stamford paid for personnel to
draw the blood for both commercial and federal patients, fill out applicable paperwork, and ship
the blood specimens to the laboratories to perform the testing.
338. To recruit HCPs to order the laboratory testing, Stamford paid commissions to
BenefitPro. BenefitPro and its recruiters then kicked back a portion of the commissions to referring
HCPs, disguising the remuneration as MSO distribution payments but was actually paid to induce
the HCPs’ commercial and Medicare, Medicaid, and TRICARE referrals to Stamford, THD, and
BHD for laboratory testing.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 79 of 154 PageID #: 962
80
1. Stamford Funded the Kickbacks Paid by BenefitPro and Regal MSOs
339. To fund the MSO kickbacks to HCPs, Stamford paid BenefitPro 25% of its net
collections for diagnostic services, including toxicology and laboratory testing, pursuant to an
agreement that Hickman and Stamford’s CEO signed on or about June 2, 2016.
340. From in or about November 2016 to November 2017, Stamford paid BenefitPro
over $7.1 million.
341. To further the Stamford kickback scheme, Hickman founded, owned, and operated
numerous corporate entities. He created BenefitPro to receive payments from Stamford and make
payments to himself, the MSOs, Gonzales, and Kash. In addition, Hickman transferred funds from
BenefitPro to APM to pay himself and others.
342. Hickman created multiple MSOs to receive payments from BenefitPro, to pay MSO
recruiters like Gonzales and Kash, and to pay referring HCPs. Those MSOs were named Cygnus
MG LLC, Eridanus MG LLC, Geminorium MG LLC, Herculis MG LLC, Indus MG LLC, Juka
MG LLC, and Korvus MG LLC (collectively, BenefitPro MSOs).
343. In or about August 2016, BenefitPro recruiters, including Gonzales, Howard, and
Kash, began implementing the BenefitPro MSO kickback scheme, targeting HCPs, offering
kickbacks, and coordinating with Stamford and its personnel.
344. In addition, Hickman agreed on behalf of BenefitPro to pay other MSO recruiters,
including Regal Health Solutions LLC (Regal), to recruit HCPs to order laboratory testing from
Stamford, THD, and BHD. BenefitPro kicked back to those MSO recruiters, including Regal, a
portion of the money that Stamford paid to BenefitPro. The MSO recruiters, in turn, kicked back
to the referring HCPs a portion of the money that the recruiters had received from BenefitPro.
345. During the period of about November 2016 to October 2017, BenefitPro paid over
$750,000 to Regal, a Texas company that Marioni and Perkins owned and operated. Through
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 80 of 154 PageID #: 963
81
numerous MSOs, Regal kicked back a portion of those payments to referring HCPs. Those MSOs
included Buena Vista Partners (Buena Vista), CHP Catalyst Health Partners LP (CHP),
Transparity Association LP (Transparity), and Transcend Partners LP (Transcend) (collectively,
Regal MSOs).
346. In or about November 2016, Regal recruiters, including Marioni and Perkins, began
implementing the Regal MSO kickback scheme, targeting HCPs, offering kickbacks, and
coordinating with Stamford and its personnel.
347. In their sales pitches to HCPs, the BenefitPro and Regal MSO recruiters focused on
the amount of money that HCPs would receive.
348. At least 25 HCPs who participated in the Stamford fraud scheme knew about MSO
kickbacks from prior experience.
349. Of those, at least nineteen HCPs who had received kickbacks from Ascend MSO
in the LRH kickback scheme also received kickbacks from the BenefitPro MSOs in the Stamford
kickback scheme. Those HCPs included Azim Karim, Thuy Nguyen, Linh Ba Nguyen, Baxter
Montgomery, Bruce Maniet, Doyce Cartrett, Elizabeth Seymour, Frederick Brown, Paul
Gerstenberg, Heriberto Salinas, Hong Davis, Huy Chi Nguyen, James Froelich, III, Muhammad
Akram Khan, Dung Hoy Nguyen, Dung Chi Nguyen, Paul Worrell, Robert Megna, and Jill Taylor.
350. In addition, at least four HCPs who had received kickbacks from Next Level MSOs
in the LRH kickback scheme also received kickbacks from the Regal MSOs in the Stamford
kickback scheme. Those HCPs included Annie Varughese, Ashley Chin, Kozhaya Sokhon, and
Tommy Pham.
351. The purpose of the Stamford MSO scheme was to pay HCPs for their referrals, as
those involved knew. For example, Stamford’s COO, who reported to the CEO and regularly
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 81 of 154 PageID #: 964

82
interacted with participating HCPs, BenefitPro, Regal, THD, and BHD, described the arrangement
in May 2016 as a “joint venture” involving “blood draws, toxicology screens (urine) and sleep
studies and EEGs,” where the “doctors get paid through a Managed Service Organization (MSO).”
352. The COO later acknowledged to colleagues that “the doctors like us and appreciate
the level of customer service we provide, but they are all about the money and who can give them
the most.” The COO noted that “the longer we participate in this program I realize it is all about
who can give them the most as many of them can’t make ends meet with their current practice
models. They are independent of their local hospitals and many of them struggle financially. So
they look for programs like this to give them additional income.” The COO noted that
“unfortunately the doctors follow the money.”
353. By following the money, the HCPs who agreed to participate in the Stamford fraud
scheme received significant sums.
BenefitPro MSOs
354. The below chart summarizes over $2 million in BenefitPro MSO payments from in
or about November 2016 to February 2018 to referring HCPs for their laboratory referrals:
HCP BenefitPro MSO MSO Payments
Amitabh Skukla Cygnus $69,169.00
Azim Karim
Cygnus; Indus
$18,364.00
Baxter Montgomery Indus $90,913.97
Bruce E. Maniet Herculis $38,847.34
Camilo Paredes
Korvus
$1,475.91
David Palombo
Cygnus
$4,744.00
Doyce Cartrett Eridanus $234,314.95
Dung Hoy Nguyen and Dung Chi Nguyen Geminorium $49,989.87
Earl Martin
Korvus
$4,245.91
Elias Ntsoane Indus $1,000.00
Elizabeth Seymour Eridanus $232,314.95
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 82 of 154 PageID #: 965

83
355. To disguise the kickbacks, Hickman and O’Neal used purported “investment”
documentation for the BenefitPro MSOs. Only HCPs who referred diagnostic services, including
laboratory testing, as part of the Stamford scheme were allowed to participate and remain in the
BenefitPro MSOs, and the only source of revenue for the BenefitPro MSOs came from the referrals
or other business generated by the HCPs in the MSOs. While HCPs purported to invest in the
BenefitPro MSOs, the MSOs’ payments to HCPs were not based on the returns from any genuine
Emen Udonta
Indus
$1,000.00
Emile Kettle
Cygnus
$1,435.00
Forbes Barnwell Herculis $4,164.83
Frederick Brown Indus $92,263.97
Graceland Investments
Geminorium
$9,314.88
Heriberto Salinas Herculis $19,322.32
Hong Davis Herculis $16,900.00
Huy Chi Nguyen
Geminorium
$49,989.87
Jacinto Medical Group Juka $124,145.39
James Froelich III Herculis $38,847.34
Jeffrey Guillory Indus $66,485.10
Jill Taylor
Indus
$152,369.90
Joseph Bolin Herculis $38,847.34
Joy Touchstone Korvus $5,312.06
Louis Coates
Herculis
$43,847.34
Louis Zegarelli Herculis $42,847.34
Lyndon Forbes Barnwell Eridanus $17,860.95
Michelle Legall
Cygnus
$5,144.00
Muhammad Akram Khan Herculis $38,847.34
Paul Gerstenberg Indus $196,501.78
Paul Worrell
Eridanus
$132,217.90
Raja Abusharr
Cygnus; Korvus
$26,199.91
Robert Megna Geminorium $112,423.71
Rosemary Bates Cygnus; Korvus $9,445.53
Thuy Nguyen and Linh Ba Nguyen
Geminorium
$90,664.85
Willie Villarreal Korvus $5,745.91
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 83 of 154 PageID #: 966
84
investment. Instead, the BenefitPro MSOs’ payments to HCPs were simply profits shared with
HCPs based on the HCPs’ referrals as part of the Stamford scheme.
356. As an example, in or about August 2016, BenefitPro recruited Physician E, of
Denton, Texas, to refer laboratory testing to BHD as part of the Stamford scheme. To induce
Physician E’s referrals to BHD, Gonzales and Howard offered to pay MSO kickbacks to Physician
E through Eradinus MSO. Physician E agreed to participate in the kickback scheme, provided
Gonzales with a purported investment check of $6,000 dated August 1, 2016, and began referring
laboratory tests for federal healthcare beneficiaries to BHD on or about August 5, 2016.
357. To eliminate any financial risk for Physician E, Hickman held Physician E’s $6,000
check without depositing it until he and Gonzales had already given her a much larger MSO
payment check. Hickman did not deposit Physician E’s check until on or about December 21,
2016. By that date, Physician E had made dozens of referrals to BHD for laboratory testing, and
Hickman had signed and Gonzales had provided to Physician E a check dated November 23, 2016
for $15,000—two and a half times the amount of Physician E’s purported investment. Moreover,
within days of Hickman depositing Physician E’s check, Hickman signed and Gonzales provided
to Physician E another MSO payment check, dated December 22, 2016, for $18,000.
358. During the period of August 2016 to January 2018, Eridanus MSO paid Physician
E over $232,000 in MSO kickbacks, a return of over 3,766% based on Physician E’s purported
investment of $6,000.
359. From in or about August 2016 to January 2018, during her participation in the
Eradinus MSO kickback scheme, Physician E referred to BHD hundreds of tests payable by federal
healthcare programs. BHD submitted those claims to federal programs, including Medicare, which
paid over $345,000 to BHD. Examples of those claims are included in Exhibit A hereto.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 84 of 154 PageID #: 967
85
360. As another example, in or about July 2016, BenefitPro recruited Doyce Cartrett,
M.D. (Physician H), of Silsbee, Texas, to refer laboratory testing to THD as part of the Stamford
scheme. To induce Physician H’s referrals to THD, Kash offered to pay MSO kickbacks to
Physician H through Eradinus MSO. Physician H agreed to participate in the kickback scheme,
provided Kash with a purported investment check of $6,000, and began referring laboratory tests
for federal healthcare beneficiaries to THD on or about September 27, 2016.
361. To eliminate any financial risk for Physician H, Hickman held Physician H’s
$6,000 check without depositing it until he and Kash had already provided Physician H with a
much larger MSO payment check. Hickman did not deposit Physician H’s check until on or about
November 28, 2016. By that date, Physician H had made dozens of referrals to THD for laboratory
testing, and Hickman had signed and Kash had provided to Physician H a check dated November
23, 2016 for $15,000—two and a half times the amount of Physician H’s purported investment.
362. During the period of September 2016 to December 2017, Eridanus MSO paid
Physician H over $234,000 in MSO kickbacks, a return of over 3,800% based on Physician H’s
purported investment of $6,000.
363. From in or about September 2016 to December 2017, during his participation in the
Eradinus MSO kickback scheme, Physician H referred to THD hundreds of tests payable by federal
healthcare programs. THD submitted those claims to federal programs, including Medicare, which
paid over $199,000 to THD. Examples of those claims are included in Exhibit A hereto.
364. In addition to paying the referring HCPs, BenefitPro paid a significant portion of
the money to Hickman, Kash, and Gonzales.
365. At Hickman’s direction, BenefitPro transferred over $1.5 million to APM, which
paid Hickman’s company, Hickman Tax and Retirement Advisors, $356,699.92 in 2017.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 85 of 154 PageID #: 968

86
366. To hide his role in the Stamford kickback scheme, Kash directed BenefitPro to pay
him through Tigerlily. From December 2016 to December 2017, BenefitPro paid Kash, through
Tigerlily, a total of $671,039.66.
367. To pay Gonzales, and in turn Howard, BenefitPro paid Zalegon $702,784.61.
Gonzales deposited the checks and paid Howard about $10,000 in cash per month, except for
December 2016, when Gonzales paid Howard about $70,000 in cash.
368. As agreed with Howard, Gonzales deposited the checks he received from
BenefitPro and withdrew cash to share with Howard. Approximately monthly, from in or about
January to November 2017, Gonzales delivered to Howard a bag of about $10,000 in cash per
month. Each month, after Howard received the bag of cash from Gonzales, she placed it in the safe
in her home, with the cash still in the bag. In total, Gonzales paid Howard about $110,000 in cash
from BenefitPro.
Regal MSOs
369. Through the Regal MSOs, Marioni and Perkins paid referring HCPs to induce their
referrals to Stamford, THD, and BHD. The below chart summarizes Regal MSOs’ payments of
over $300,000 to the following referring HCPs to induce their referrals:
HCP
MSO
MSO Payments
Andres Mesa Transparity $25,742
Annie Varughese Transparity $28,280
Ashley Chin CHP $17,390
Asif Ali
Transparity
$16,586
Chhay Tay CHP $8,195
Harish Thakkar CHP $8,592
Joy Touchstone
Transparity
$2,400
Karan Bhalla CHP $43,475
Kozhaya Sokhon Transparity $33,172
Mike Rodriguez
Transparity
$33,172
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 86 of 154 PageID #: 969

87
HCP MSO MSO Payments
Orlando Kypuros Transparity $5,039
Raja Abusharr
Transcend
$2,900
Rishi Hingorani Transparity $6,506
Russel Vanbiber Transparity $13,362
Syed Yusoof
Buena Vista
$3,715
Tanya Grun Transcend $2,800
Tommy Pham CHP $58,009
370. To disguise the kickbacks, Marioni and Perkins used purported “investment”
documentation for the Regal MSOs. Only HCPs who referred diagnostic services, including
laboratory testing, as part of the Stamford scheme were allowed to participate and remain in the
Regal MSOs, and the only source of revenue for the Regal MSOs came from the referrals or other
business generated by the HCPs in the MSOs. While HCPs purported to invest in the Regal MSOs,
the MSOs’ payments to HCPs were not based on the returns from any genuine investment. Instead,
the Regal MSOs’ payments to HCPs were simply profits shared with HCPs based on the HCPs’
referrals as part of the Stamford scheme.
371. As an example, in or about December 2016, Regal recruited Kozhaya Sokhon, M.D.
(Physician I), of Houston, Texas, to refer laboratory testing to BHD as part of the Stamford scheme.
To induce Physician I’s referrals, Perkins and Marioni offered to pay MSO kickbacks to Physician
I through Transparity MSO. Physician I agreed to participate in the kickback scheme and began
referring laboratory tests for federal healthcare beneficiaries to BHD on or about December 6,
2016.
372. During the period of about December 2016 to at least October 2017, Transparity
MSO paid Physician I over $33,000 in MSO kickbacks, a return of over 450% based on his
purported investment of $6,000.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 87 of 154 PageID #: 970
88
373. From in or about December 2016 to September 2017, during Physician I’s
participation in the Transparity MSO kickback scheme, Physician I referred to BHD hundreds of
tests payable by federal healthcare programs. BHD submitted those claims to federal programs,
including Medicare, which paid over $22,000 to BHD. Examples of those claims are included in
Exhibit A hereto.
2. Stamford, BenefitPro, and Regal Partnered with THD and BHD
374. As part of the Stamford fraud scheme, THD and BHD agreed with Stamford,
BenefitPro, and Regal that the laboratories would bill federal healthcare programs for the resulting
referrals of laboratory testing for federal healthcare program beneficiaries. Grottenthaler and Kash
agreed to this approach on behalf of THD, and Theiler agreed on behalf of BHD.
375. Grottenthaler, Kash, and Theiler understood that Stamford was concerned about the
legality of billing federal healthcare programs for claims referred by MSO participants. The
laboratory executives agreed to bill those claims to capture the lucrative revenue from federal
healthcare program referrals.
376. As agreed among the parties, Stamford paid for phlebotomists or other medical staff
located in the offices of HCPs who were receiving MSO kickbacks. Often, those phlebotomists
had previously worked in the particular HCPs’ offices.
377. For example, Stamford paid Lacrimioara Hurgoiu, who was already working in Dr.
Annie Varughese’s office as a registered nurse, $18 per hour for 30-40 hours a week to draw blood
for laboratory tests that Varughese referred to Stamford, THD, and BHD. Stamford paid Tracy
Tompkins, who was already working in Dr. Elizabeth Seymour’s office as a phlebotomist, $19 per
hour for 30-40 hours a week to draw blood for laboratory tests that Seymour referred to Stamford,
THD, and BHD.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 88 of 154 PageID #: 971
89
378. Stamford paid the phlebotomists to draw the blood for patients with federal
healthcare insurance and patients with commercial insurance. Stamford, BenefitPro, Regal, THD,
and BHD instructed the phlebotomists to collect insurance information for federal healthcare
beneficiaries and provide that information to THD or BHD, so that THD or BHD could bill for the
claims.
379. To ensure that they would receive the federal referrals from the Stamford kickback
scheme, THD and BHD provided the phlebotomists with supplies for the blood specimens,
laboratory-specific requisition forms, and laboratory-specific shipping materials.
380. Stamford, BenefitPro, Regal, THD, and BHD instructed the phlebotomists to use
the THD or BHD requisition forms and shipping materials for federal healthcare program
beneficiaries. Following those instructions was important to THD and BHD so that they could bill
the resulting federal claims.
381. To ensure the success of the Stamford kickback scheme, and at the direction of
Grottenthaler, Kash, and Theiler, THD and BHD identified HCP targets for the BenefitPro and
Regal MSOs, referred HCPs interested in kickback payments to the BenefitPro and Regal MSOs
to secure their blood testing referrals, participated with the MSOs in sales pitches to offer HCPs
money to induce their referrals, and sought to ensure that they would receive the federal referrals
resulting from the kickbacks.
382. In or about November 2016, Grottenthaler and Kash learned that some HCPs
referring to Stamford had not sent the federal payer information to THD. Kash reminded Stamford
that the federal payer information needed to be sent to THD with the blood specimens.
383. After Kash’s reminder, Stamford’s COO contacted BenefitPro and multiple
Stamford employees responsible for interacting with the phlebotomists and HCPs, advising them
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 89 of 154 PageID #: 972
90
that “we are receiving feedback [from Kash] that some of the clinics are listing the ‘Federal Payers’
on their requisition logs but not providing the payer (insurance info) so [THD] can bill for it.”
Stamford’s COO stated: “We need to ensure that all of our clinics are listing the federal payors on
the requisition forms and providing insurance cards etc.” Stamford’s COO instructed the Stamford
employees to contact “all clinics reminding them that if there is a federal payor, i.e. Medicare,
Medicaid, Blue Cross Blue Shield Federal; Champus, Tricare etc. that they must include the payer
information for billing purposes for [THD].” Stamford’s COO clarified that this policy to collect
“all federal payor info” applied for each “lab who is billing for the service.”
384. In addition, Stamford’s COO asked BenefitPro to “alert[] your reps that all payer
info must be provided” and to “remind the phelbs [phlebotomists] we draw for the federal payers,
in their clinics, but the lab is responsible for billing the federal payors and they will need that info.”
385. In or about February 2017, after THD had billed the federal claims resulting from
the Stamford arrangement for about nine months, THD considered, for compliance reasons,
ceasing its role in billing the resulting federal claims. That month, Grottenthaler told Stamford’s
CEO that THD eventually wanted to stop billing the resulting federal claims. Stamford’s CEO told
Grottenthaler and O’Neal that while previously “these [federal] payors were billed by [THD],”
“our position on this has not changed and Stamford will not bill these federal payors.” As Stamford
and THD knew from experience and HHS-OIG’s June 2014 Special Fraud Alert, HCPs typically
wish to minimize the number of laboratories to which they refer for reasons of convenience and
administrative efficiency. Stamford’s CEO told Grottenthaler that Stamford would “find another
lab partner for this aspect of our service” “[i]f THD chooses not to bill for these patients going
forward.”
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 90 of 154 PageID #: 973
91
386. Grottenthaler and THD delayed making the change for months. On or about May
25, 2017, THD purported to stop billing the federal claims resulting from the Stamford
arrangement. Stamford informed its employees of the change to “no more federal” referrals for
THD, noting that “this is a [THD] move – not ours.” Stamford’s COO asked Stamford employees
and phlebotomists to alert HCPs who ordered THD tests that “effective immediately we will not
be able to continue drawing specimens, for Federal Payers, and then sent [sic] to [THD] Laboratory
in Richmond VA or receive them in the Stamford Memorial Hospital on site lab.”
387. THD’s decision to stop submitting the federal claims resulting from the Stamford
scheme did not last long. Less than two months later, Grottenthaler decided to reinstate THD’s
prior policy of billing the resulting federal claims. Grottenthaler was aware of how much money
THD could lose by not billing those federal claims.
388. On or about July 14, 2017, as a result of Grottenthaler’s decision, THD told
Stamford phlebotomists to draw blood specimens for federal healthcare beneficiaries. To ensure
THD would receive the federal referrals resulting from the Stamford kickback scheme, THD
provided the phlebotomists with THD requisition forms and labels to ship the specimens to THD.
389. On or about the same day, Kash confirmed THD’s change in position to Stamford’s
CEO and COO and O’Neal. Kash noted that THD had learned that Stamford was sending blood
specimens for federal patients in the Dallas, Houston, and El Paso areas to BHD (THD’s
competitor). Kash also referenced THD’s awareness that not billing the resulting federal claims
could lead to a broader loss of business, noting that at least one HCP had “left the [Stamford]
hospital relationship because of not being able to draw blood on all her patients.”
390. As intended by Grottenthaler and Kash, THD’s participation with Stamford and
others in the MSO scheme was lucrative, with Stamford paying THD over $9.5 million.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 91 of 154 PageID #: 974
92
391. As intended by Theiler, BHD’s participation with Stamford and others in the MSO
scheme was lucrative, with Stamford paying BHD over $7.5 million.
III. ADDITIONAL THD KICKBACK SCHEMES
392. Aware of the financial success and astronomical growth of a prior laboratory known
as HDL, Grottenthaler sought to adopt the illegal practices used by HDL.
A. At THD, Grottenthaler Sought to Replicate HDL’s Kickback-Fueled Growth
1. HDL Used Kickbacks to Gain Business
393. HDL was a clinical laboratory that offered blood tests for cardiovascular disease
and diabetes, and it sought to persuade doctors to order HDL’s tests for their patients.
394. In 2010, HDL agreed to pay volume-based commissions to BlueWave Healthcare
Consultants, Inc. (BlueWave) to arrange for and recommend that HCPs order laboratory testing
from HDL. In turn, BlueWave paid volume-based commissions to its independent-contractor
marketers (collectively, BlueWave marketers).
395. To generate laboratory testing referrals, HDL and BlueWave agreed to a kickback
scheme in which HDL would pay, and BlueWave marketers would offer, to HCPs a $3 “draw fee”
(ostensibly as compensation for drawing patients’ blood), plus a $17 “processing and handling”
(P&H) fee (ostensibly as compensation for handling blood samples), for a total of $20 per patient
that the HCPs referred for HDL laboratory testing. HDL and BlueWave believed this financial
inducement was “a critical door opener” with HCPs.
396. In mid-2010, while engaged in the P&H fee kickback scheme with HDL, BlueWave
agreed to participate in a kickback scheme with another laboratory, Singulex, Inc., in which
Singulex would pay HCPs a $10 P&H fee per patient that the HCP referred for Singulex laboratory
testing.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 92 of 154 PageID #: 975
93
397. In selling HDL and Singulex laboratory tests, BlueWave marketers emphasized to
doctors the money to be made from P&H fees. BlueWave marketers targeted “money hungry”
doctors and used P&H fees as an inducement. BlueWave marketers even showed HCPs “pro
formas” with calculations of how much money the HCPs could receive by ordering HDL and
Singulex laboratory tests.
398. Using kickbacks to drive business, BlueWave grew HDL’s business from fewer
than 100 Medicare patient referrals in 2009, to over 40,000 in 2009, over 120,000 in 2011, and
over 200,000 per year from 2012 to 2014.
399. On June 25, 2014, as noted above, HHS-OIG issued a Special Fraud Alert warning
that P&H fee payments to physicians or physician practices “present a substantial risk of fraud and
abuse” and “are suspect under the [AKS].” 79 Fed. Reg. 40,114, 40,116 (2014).
400. Referrals to HDL dropped significantly after it stopped paying P&H fees to HCPs,
and HDL declared bankruptcy less than a year later.
401. On January 9, 2015, HDL ended its sales agreement with BlueWave, citing
compliance concerns. HDL sued the BlueWave founders on January 13, 2015 in the U.S. District
Court for the Eastern District of Virginia, alleging that the BlueWave founders had “put their
economic interests ahead of compliance.”
402. On January 29, 2015, Singulex settled FCA allegations that, among other things, it
had paid P&H fee kickbacks to induce referrals for laboratory testing reimbursed by federal
healthcare programs in violation of the AKS, and had submitted claims for medically unnecessary
laboratory tests, from January 1, 2010 to October 31, 2014.
403. On March 31, 2015, HDL agreed to pay $47 million plus additional contingent
payments up to $100 million to settle FCA allegations that, among other things, it had paid P&H
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 93 of 154 PageID #: 976
94
fee kickbacks to induce referrals for laboratory testing reimbursed by federal healthcare programs
in violation of the AKS, and had submitted claims for medically unnecessary laboratory tests, from
November 25, 2008 to January 31, 2015.
404. On April 9, 2015, the U.S. District Court for the District of South Carolina granted
the United States’ motion to intervene in a qui tam suit against BlueWave and its founders and
HDL’s former CEO alleging that defendants had offered or paid “kickbacks to referring physicians
disguised as ‘process and handling’ fees,” had submitted or caused to be submitted claims for
medically unnecessary laboratory tests, and had conspired to violate the FCA.
405. On August 7, 2015, the United States filed its complaint in intervention against
BlueWave and its founders and HDL’s former CEO. After a twelve-day trial, the jury returned a
unanimous verdict on January 31, 2018, finding that the BlueWave founders and former HDL
CEO had violated the FCA. Judgment was entered against the BlueWave founders in the amount
of $114,148,661.86 and against the former HDL CEO in the amount of $111,109,655.30. The
United States Court of Appeals for the Fourth Circuit affirmed the judgment in all respects, and
the Supreme Court denied defendant’s petition for a writ of certiorari. United States v. Mallory,
988 F.3d 730 (4th Cir. 2021), cert. denied, 2021 WL 5284633 (Nov. 15, 2021).
2. As Grottenthaler Intended, THD Used Kickbacks to Gain Business
406. Grottenthaler founded THD and registered the company with the Texas Secretary
of State in July 2014. He was THD’s CEO from its founding in 2014 until its bankruptcy in 2019.
407. In building THD’s business, Grottenthaler chose to seek and rely on payments from
federal healthcare programs, including Medicare, Medicaid, and TRICARE.
408. THD received its National Provider Identifier (NPI) from CMS in August 2014.
409. In 2014, THD had fewer than ten physician clients, little business, and received less
than $25,000 in Medicare payments.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 94 of 154 PageID #: 977
95
410. Grottenthaler knew of HDL’s astronomical growth and sought to replicate its
business model. Immediately after HDL terminated its sales agreement with BlueWave on January
9, 2015, citing compliance concerns, Grottenthaler contacted BlueWave marketers to offer them
jobs as THD sales managers and employees. Grottenthaler hired over a dozen BlueWave marketers
in January 2015 and hired at least six more BlueWave marketers the following month.
411. When he offered sales jobs to BlueWave marketers in early 2015, Grottenthaler
knew that the BlueWave marketers were alleged to have offered kickbacks to HCPs to induce their
referrals. During the P&H fee kickback scheme, the BlueWave marketers were well-known in the
laboratory industry for using financial inducements to get business. For example, on September 8,
2014, the Wall Street Journal published a Page One article by John Carreyrou and Tom McGinty
titled, “Medicare Unmasked: A Fast-Growing Medical Lab Tests Anti-Kickback Law.” The
article described the P&H fee kickback scheme in which HDL “paid doctors who sent it patients’
blood for testing,” identified BlueWave as the “independent sales-and-marketing contractor,” and
highlighted BlueWave’s position that “‘this is an ph fee not a draw fee. One word makes it legal
the other illegal.’”
412. Grottenthaler chose to hire BlueWave marketers to leverage their relationships with
the same HCPs who had received kickbacks from HDL and Singulex.
413. Grottenthaler did not rethink his business strategy after the Department of Justice
announced millions of dollars in settlements due to BlueWave’s kickbacks. Nor did he change his
strategy after the Department of Justice intervened and filed a complaint in the FCA case against
the BlueWave founders and HDL CEO.
414. Instead, Grottenthaler doubled down on his plan to replicate HDL’s business model,
and THD purchased HDL’s assets for $37 million in a bankruptcy auction in September 2015.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 95 of 154 PageID #: 978
96
415. Shortly after the acquisition became public, Grottenthaler wrote letters to HDL’s
referring HCPs—many of whom he knew had received P&H fee kickbacks from HDL—promising
that it was “business as usual” and that “[THD] and [HDL] have shared values with regard to the
importance of compliance with federal and state regulatory requirements in connection with
oversight and management of its business operations.”
416. In addition, throughout 2015, Grottenthaler employed BlueWave marketers as
THD’s sales management and employees, and even hired additional BlueWave marketers,
ultimately hiring more than 35 for sales roles at THD.
417. Because of Grottenthaler’s focus on hiring BlueWave marketers, at least 36 of
THD’s 45 sales employees (80%) in 2015 were BlueWave marketers.
418. Grottenthaler also placed BlueWave marketers in sales leadership positions. Each
of the three VPs of Sales he hired in 2015 were BlueWave marketers. Grottenthaler hired Cornwell
as the VP of Sales for the Southwestern Region, with responsibility for sales in Texas. Cornwell
reported directly to Grottenthaler. Love was hired as a THD Account Executive in January 2015,
and she reported to Cornwell.
419. Once Grottenthaler hired the BlueWave marketers, he expected them to conduct
“business as usual.” One of THD’s VP of Sales emphasized that approach to THD’s sales
personnel in October 2015, copying Grottenthaler and stating “All [HDL] accounts will notice
nothing different. HDL business as usual. All [HDL] tests available as normal.”
420. Following Grottenthaler, THD’s sales personnel pitched THD to HDL’s referring
HCPs as the new HDL, HDL 2.0, or “True HDL.” THD’s Senior VP of Sales and Marketing
echoed this theme in a THD sales call script sent to Grottenthaler in October 2015. THD advised
its sales personnel to tell HDL’s referring HCPs that “I would like to assure your office that things
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 96 of 154 PageID #: 979
97
will continue business as usual. Everything from the requisition you utilize, the way the patient
specimen is sent, received and processed, to the color-coded patient report and billing policies will
remain the same.”
421. Aware of the importance of Medicare payments to THD’s business, THD advised
its sales personnel to tell HDL’s referring HCPs that “[THD] does have a Medicare and CLIA #
which can and should be utilized.”
422. As planned by Grottenthaler, the BlueWave marketers at THD marketed many of
the same laboratory tests as HDL to many of the same HCPs who had previously received P&H
fee kickbacks. Burt Lively, a former BlueWave marketer that Grottenthaler hired as a THD VP of
Sales, testified that HDL’s and THD’s tests were “almost identical” and that “almost all” of THD’s
clients were “former HDL clients.” Tony Carnaggio, a BlueWave marketer turned THD sales
representative, testified that a lot of the doctors that ordered HDL tests later ordered the same tests
from THD. Erika Guest, another BlueWave marketer turned THD sales representative, testified
that THD was “marketing all of the tests that HDL had.”
423. Grottenthaler developed and negotiated the compensation packages for the
BlueWave marketers. At Grottenthaler’s direction, THD offered them significant financial
incentives to secure HCP referrals to THD. Those incentives included commissions based on net
collected reimbursements and various bonuses, including for new physician or hospital business.
424. Grottenthaler also was highly motivated to quickly grow THD’s business.
Grottenthaler sought to, and ultimately did, pay himself large salaries, bonuses, and shareholder
distributions.
425. To quickly increase HCP referrals to THD, Grottenthaler agreed that THD would
implement at least four kickback schemes, involving: (1) P&H fees; (2) waivers of patient
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 97 of 154 PageID #: 980
98
copayments and deductibles; (3) consulting fees; and (4) MSO payments. THD and its co-
conspirators then submitted claims to federal healthcare programs for the tests resulting from the
kickbacks and improper financial relationships.
426. To capitalize on the financial success that THD experienced due to the fraud
schemes, Grottenthaler sought to cash in before THD faced punishment for those schemes. In or
about early 2016, Grottenthaler launched efforts to recapitalize or sell THD, which concluded with
a recapitalization transaction on or about January 26, 2017 in which Grottenthaler received $36.95
million.
B. THD’s P&H Fee Kickback Scheme
427. After the June 2014 Special Fraud Alert, HDL stopped paying P&H fees directly
to HCPs. Instead, HDL paid P&H fees to draw site companies that were purportedly independent
of referring HCPs. In fact, numerous companies were simply conduits to pay P&H fees to HCPs
to induce referrals for laboratory testing.
428. Grottenthaler, Cornwell, and Love knew that the P&H fees would induce HCP
referrals for laboratory testing.
429. After acquiring HDL, Grottenthaler continued HDL’s practice of paying P&H fees
to purported draw site companies. In a letter he sent in or about October 2015 to the draw site
companies that HDL had paid, Grottenthaler said that THD “remain[s] fully committed to our
draw site partner relationships.” Grottenthaler promised the draw site companies that THD would
send them an updated agreement to pay them P&H fees, and “[t]he agreement will have no
substantive changes to the contract originally in place with [HDL], however it will reflect the new
company name.”
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 98 of 154 PageID #: 981
99
1. Oakmont Wellness Kickback Scheme
430. Oakmont Wellness Center, PA (Oakmont), located in Fort Worth, Texas, was a
family medicine clinic formed in or about January 2011. Bibi Tasleyma Sattar, D.O. (Physician J)
was Oakmont’s sole owner and physician.
431. Physician J and Oakmont were enrolled as HCPs with Medicare, Medicaid, and
TRICARE. Oakmont’s patients included beneficiaries of those federal programs.
432. Physician J’s father, Sultan Satar Sattar (Sultan), was Oakmont’s office manager.
Physician J’s mother, Bibi Zabeda Sattar (Zabeda), was Oakmont’s receptionist and referral
coordinator. Sultan and Physician J controlled Oakmont’s bank account.
433. In or about July 2013, Cornwell, as a BlueWave marketer, offered P&H fee
kickbacks to Physician J and Oakmont to induce their laboratory referrals to HDL and Singulex.
434. From July 2013 to July 2014, HDL and Singulex paid over $45,000 in P&H fee
kickbacks to Physician J and Oakmont.
2. Total Health Kickback Scheme
435. About a month after the June 2014 Special Fraud Alert on P&H fee payments,
Zabeda formed Total Health Diagnostics, Inc. (Total Health), a Texas corporation.
436. Zabeda formed Total Health to receive P&H fee kickbacks from HDL.
437. Zabeda served as a director of Total Health. The company’s address was a residence
in Mesquite, Texas, belonging to Sultan and Zabeda’s son (Physician J’s brother).
438. Shortly after forming Total Health, HDL and Total Health finalized an agreement
for HDL to pay P&H fees to Total Health to induce Physician J’s and Oakmont’s referrals to HDL
for laboratory testing. Zabeda signed the agreement on behalf of Total Health.
439. Cornwell and Love knew that Sultan and Zabeda were Physician J’s
parents, worked for Oakmont, and had a financial interest in Total Health.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 99 of 154 PageID #: 982
100
440. In October 2014, Zabeda emailed Cornwell and Love demanding that HDL pay
P&H fees to Total Health, threatening that “if payment to Total Health Diagnostics is not received
by October 6, 2014, we will be forced to discontinue all blood draw that is sent to your lab.”
441. The same day, Cornwell forwarded Total Health’s demand for P&H fees to HDL.
In response, HDL’s chief compliance officer told Cornwell that HDL “cannot pay P&H” to Total
Health and directed Cornwell to read an email from her compliance employee, who noted that “the
agreements and W-9’s for Total Health Diagnostics are signed by a Bibi Zabeda Sattar.” In
response, one of BlueWave’s founders told Cornwell that day that “I wouldn’t touch [the Total
Health arrangement] with a ten foot pole because it’s an immediate family member and the
perception/argument would be Money could flow back to referring provider.”
442. Cornwell then corresponded with Love and others about the Total Health
arrangement. Referring to the Total Health P&H fee agreement, Cornwell told Love that
“[a]ccording to [HDL’s chief compliance officer], ‘all’ attorneys agree that it shouldn’t be
honored.” A colleague asked, “Why can’t she [Physician J] just draw in office herself?” Cornwell
responded to the group, including Love, “Because Sultan wants to get paid for it.”
3. ODS Kickback Scheme at Oakmont
443. Grottenthaler hired Cornwell and Love to join THD’s sales force in or about
January 2015. At Grottenthaler’s direction, THD paid Cornwell and Love to arrange for and
recommend referrals to THD for federal healthcare business, including referrals from Oakmont.
444. In or about April 2015, Cornwell contacted Sultan to ensure that Physician J and
Oakmont would order THD laboratory tests for patients, including federal beneficiaries. As
Cornwell and Love knew, Sultan wanted to get paid for the referrals to THD.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 100 of 154 PageID #: 983
101
445. In or about April 2015, Zabeda formed Onsite Draw Station, Inc. (ODS), a Texas
corporation. Zabeda served as ODS’s director and controlled ODS’s bank account. The company’s
address was Physician J’s residence in Fort Worth, Texas, where Sultan and Zabeda also resided.
446. In or about April 2015, Love went to Oakmont’s office to pick up taxpayer
identification forms for THD to set up a compensation arrangement with ODS. Love then asked
Zabeda to provide agreements with other laboratories so that Love could “really help expedite”
approval of the THD compensation arrangement.
447. In or about May 2015, THD and ODS entered into an agreement for THD to pay
ODS P&H fees of “$25.00 per specimen collected and sent by [ODS] to [THD] for testing.”
448. In the THD P&H fee agreement, ODS listed its address as Physician J’s residence
in Fort Worth, Texas, where Sultan and Zabeda also resided.
449. Grottenthaler signed the agreement to pay P&H fees to ODS on or about May 26,
2015.
450. During the arrangement, ODS collected blood specimens from Physician J’s and
Oakmont’s patients; Physician J and Oakmont referred blood testing to THD; ODS sent the blood
specimens to THD for testing; Physician J’s parents, acting through ODS, invoiced THD for P&H
fees for each referral; and THD paid the P&H fee kickbacks to ODS.
451. Cornwell and Love knew that Sultan and Zabeda were Physician J’s
parents, worked for Oakmont, and had a financial interest in ODS.
452. Love advised Zabeda in June 2015 to provide a referral log to THD to be paid P&H
fees. Later that month, Zabeda submitted the referral log to THD, with an invoice to THD for $25
in P&H fees per referred specimen.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 101 of 154 PageID #: 984
102
453. In or about July 2015, Zabeda informed Cornwell and Love that her “business
email” for ODS was [email protected]. About a week later, Zabeda asked Cornwell
and Love “if there is a problem” because “we have not got payment.” Love responded the same
day, informing Zabeda that “[a] check was sent on the 15th so you should be receiving it soon.”
Love asked Zabeda to “let me know if you don’t” receive the P&H fee check from THD.
454. From in or about July to December 2015, THD paid over $35,000 in P&H fee
kickbacks to ODS to induce laboratory referrals to THD. In or about 2016, THD paid over
$110,000 in P&H fee kickbacks to ODS to induce laboratory referrals to THD. In or about 2017,
THD paid over $115,000 in P&H fee kickbacks to ODS to induce laboratory referrals to THD.
455. THD paid Physician J’s parents, through ODS, for the referral of and arranging for
healthcare business for which payment may be made in whole or in part under the Medicare,
Medicaid, and TRICARE programs.
456. Of the P&H fee kickbacks THD paid to ODS, Physician J’s parents retained
thousands of dollars of those payments for themselves. In addition, ODS kicked back to Oakmont
thousands of dollars of those payments under the guise of renting office space from Oakmont.
457. From 2015 to 2017, during the ODS kickback scheme, THD submitted claims to
Medicare, Medicaid, and TRICARE for clinical laboratory services for beneficiaries referred by
ODS and Oakmont. Those federal healthcare programs paid THD over $800,000 on those claims.
458. On January 5, 2022, Cornwell entered a guilty plea to Count 1 of the indictment in
United States v. Cornwell, No. 4:19-CR-319 (E.D. Tex.), admitting that he knowingly and willfully
conspired with one or more persons to violate the AKS in connection with THD’s payments of
P&H fees to ODS to induce Oakmont’s laboratory referrals to THD.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 102 of 154 PageID #: 985
103
C. THD’s Copayment and Deductible Waiver Kickback Scheme
459. Despite knowing that waiving patient copayments and deductibles could violate the
AKS, and aware of FCA claims against HDL for such conduct, Grottenthaler chose to mimic
HDL’s practice of routinely waiving patient cost-sharing obligations.
460. Grottenthaler knew that THD’s HCP referral targets may be “concerned their
patients will be billed too much” for THD tests, causing the patients to “complain or leav[e] [the]
physicians.” Grottenthaler also knew that THD could gain business from competitors based on
their “billing model.”
461. For patients insured by TRICARE or private insurers, Grottenthaler decided that
THD would routinely waive copayments and deductibles, in full or in part, to induce HCPs to refer
to THD their blood testing business, including the lucrative Medicare business. Grottenthaler
envisioned THD’s billing policy as a benefit to HCPs, who received an opportunity to market free
laboratory testing to their existing and prospective patients, to make their offices more attractive
to patients and increase their revenue.
462. By waiving copayments and deductibles for TRICARE- and privately-insured
patients, Grottenthaler also sought to induce patients to agree to THD testing and to buy silence
from patients who otherwise would object to their HCPs and insurers if they had to pay large bills
for unnecessary tests.
463. Grottenthaler approved internal documents to train THD’s sales force on its “billing
message”: “Most patients will not receive a bill.” For those who do, “No co-pays. 3 invoice
reminders. Patients will not be harassed by phone calls or sent to collections.”
464. In THD’s written policy, THD stated that it would make “reasonable attempts to
collect” from patients; specifically, “up to 3 statements will be sent,” with “no harassing phone
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 103 of 154 PageID #: 986
104
calls,” and patients who cannot afford the payments “due to financial hardship may qualify for an
adjustment on their bill if they call the [THD billing] number below.”
465. As Grottenthaler intended, THD’s written billing policy made it appear as if THD
was seeking to collect copayments and deductibles, while in fact reassuring HCPs that THD would
take no action besides requesting voluntary payments.
466. THD’s sales force communicated THD’s copayment and deductible waiver policy
to HCPs in their sales pitch. For example, in a pitch to a prospective HCP’s office, a THD sales
representative explained that THD would “just send[] the 3 statements,” that “patients can initiate
contact with the billing department and discuss what they can pay,” that THD would accept what
patients believe they can pay “whatever it is, even if it’s only a dollar, or nothing,” and that if the
patient did not pay, THD would “not send[] any patient to collections” but would “write it off.”
467. The THD sales representative confirmed that “[p]atients will have a bill, in the
amount that they will discuss with [THD]. If they choose to pay, they can. If they choose not to
pay, [THD] is not going to pursue it. They can pay all of it, some of it, or none of it, based on their
own assessment of their ability to pay/financial hardship. There will be no difference if they are a
federal payer, or TRICARE, or Medicare.”
468. After THD declared bankruptcy in 2019, THD’s Liquidating Trustee publicly
released additional information, previously withheld as privileged, about Grottenthaler’s role in
the copayment and deductible waiver kickback scheme. The Trustee detailed how counsel at the
law firm Stewart Dugger & Dean PLLC warned Grottenthaler in 2014 that waiving patient
copayments and deductibles could violate “both federal and state law,” and explicitly warned
“against waiving deductibles or copayments in an effort to induce patients to use the [HCP’s]
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 104 of 154 PageID #: 987
105
services.” The Trustee also described how counsel at the law firm Perkins Coie LLP warned THD
that the systematic waiver of copayments in various states was “insurance fraud” and “illegal.”
469. Grottenthaler and THD sought to mask THD’s true billing policy. THD’s Trustee
detailed how THD removed a statement from its written billing policy that “no patient will be sent
to collections” but in fact continued to follow that policy. As THD’s CFO noted internally, “we
shouldn’t put in writing whether or not we’ll send patients to collections.”
470. The Trustree detailed how THD’s accounting personnel, aware of THD’s true
policy, continued to create financial models based on THD’s “policy not to hold the patient
responsible.”
D. THD’s Consulting Fee Kickback Scheme
471. As a BlueWave marketer, Cornwell had offered P&H fee kickbacks to Jaspaul
Bhangoo, M.D. (Physician K), of Denton, Texas, in connection with the physician ordering from
HDL and Singulex. Physician K accepted Cornwell’s offers, and from 2012 to 2014, Physician K
received between $18,700 and $44,200 per year in P&H fees from HDL and additional P&H fees
from Singulex.
472. When he was receiving P&H fee kickbacks, Physician K referred a high volume of
laboratory tests to HDL and Singulex—more than 2,200 patient specimens to HDL in 2013 alone.
473. Grottenthaler hired Cornwell to recruit HDL’s referring HCPs like Physician K to
order THD laboratory tests.
474. In or about January 2015, Cornwell offered to pay Physician K purported consulting
fees of $5,000 per month to induce Physician K to order THD laboratory tests.
475. On or about January 20, 2015, Physician K cashed Cornwell’s first check. Within
a week, Physician K had signed a new account form with THD, and Cornwell told Grottenthaler
that Physician K will order THD tests for “60-70 [patients] per week beginning next week.”
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 105 of 154 PageID #: 988
106
Induced by the consulting fee kickbacks, Physician K began ordering THD tests on or about
February 2, 2015, when he referred to THD eight laboratory tests for a Medicare beneficiary. THD
submitted the claims to Medicare, which paid THD over $75. On or about February 4, 2015,
Physician K referred to THD over 20 tests for at least four patients. THD submitted the claims to
federal healthcare programs, including Medicare, which paid THD over $415.
476. For another three months, Cornwell continued paying the consulting fee kickbacks
to Physician K to induce his laboratory testing referrals to THD, Physician K continued referring
laboratory testing to THD, and THD continued submitting the claims to federal healthcare
programs.
477. On or about February 10, 2015, Cornwell paid Physician K another $5,000. That
month, Physician K referred over 345 laboratory tests to THD. THD submitted the claims to
federal healthcare programs, including Medicare, which paid THD over $14,900.
478. In or about March and April 2015, Cornwell paid Physician K another $5,000 each
month. During that period, Physician K referred over 3,500 laboratory tests to THD. THD
submitted the claims to federal healthcare programs, including Medicare, which paid THD over
$44,500.
479. Exhibit A hereto includes example claims resulting from the consulting fee
kickbacks to Physician K.
480. In or about June 2015, Cornwell recommended to Grottenthaler that Physician K
be paid as a member of a “THD Advisory Board.”
481. Grottenthaler knew that Cornwell’s HCP accounts generally, and Physician K and
other high-referring HCPs in particular, were “critical” to THD’s success.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 106 of 154 PageID #: 989
107
482. To induce Physician K’s referrals to THD, Grottenthaler arranged for THD to pay
Physician K as a purported consultant on THD’s advisory board.
483. Effective on or about August 1, 2015, THD entered into a purported consulting
agreement with Physician K for “Advisory Board Services.” The agreement purported to “engage
Consultant [Physician K] to serve on the Advisory Board,” paying him $250 per hour.
484. In fact, THD had no advisory board or advisory board members. Instead, the
purported advisory board was a cover story for Grottenthaler and Cornwell to justify paying
kickbacks to Physician K.
485. To secure Physician K’s continued referrals to THD, Grottenthaler authorized
thousands of dollars of purported advisory board consulting payments to Physician K.
486. As Grottenthaler and Cornwell knew, THD had no advisory board meetings,
agendas, or notes of any advisory board discussions. Nor did Physician K provide any other
consulting services to THD.
487. Yet, as authorized by Grottenthaler, THD paid Physician K tens of thousands of
dollars as if he were an actual advisory board consultant.
488. To disguise the kickbacks, THD documented that it paid $8,750 to Physician K in
October 2015 for his “Advisory Board hours for August and September [2015]” in which he
performed “case review” (19 hours), “new report design” (10 hours), and “review of Medicare
medical necessity” (6 hours). As Grottenthaler and Cornwell knew, Physician K did not perform
these services, much less work those hours, for THD.
489. THD further documented that it paid $4,500 to Physician K in December 2015 for
his October 2015 consulting hours spent on “case review” (9 hours), “peer to peer” discussions (6
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 107 of 154 PageID #: 990
108
hours), and “review Medicare medical necessity” (3 hours). As Grottenthaler and Cornwell knew,
Physician K did not perform these services, much less work those hours, for THD.
490. THD also documented that it paid $8,500 to Physician K in February 2016 for his
November and December 2015 “Medical/Advisory Board” consulting hours spent on “case
review” (22 hours) and four “speaker presentations” (12 hours). As Grottenthaler and Cornwell
knew, Physician K did not perform these services, much less work those hours, for THD.
491. THD’s “advisory board” consulting payments to Physician K had the effect that
Grottenthaler and Cornwell intended. From in or about August to December 2015, Physician K
referred over 12,000 laboratory tests to THD, for which Medicare paid over $189,000. From in or
about January to February 2016, Physician K referred over 5,000 laboratory tests to THD, for
which Medicare paid over $90,000. Examples of those claims are included in Exhibit A hereto.
IV. MEDICALLY UNNECESSARY TESTING
492. In each of the above kickback schemes, the kickbacks were paid to induce the HCP
recipients to routinely order large numbers of laboratory tests for screening purposes, regardless
of whether any or all of the tests were reasonable and necessary for the patient. The kickbacks had
their desired effect. HCPs ordered laboratory testing even when not reasonable and necessary, and
THD, BHD, and LRH billed federal healthcare programs for the medically unnecessary testing.
493. In addition to using kickbacks to generate more referrals, defendants arranged for
and recommended that HCPs order (a) panels of many individual tests; and (b) specific, unusual
tests with limited or no clinical utility.
494. THD and LRH offered a “Little River Assessment Panel” that consisted of many
of the same tests that THD arranged for and recommended that HCPs order from THD. The Little
River Assessment Panel included a standard lipid panel plus over 25 specialty tests, including
Apolipoprotein A1, LDL-P/HDL-P (NMR), sdLDL-C, Apolipoprotein B, HDL-2 Subclass, Lp(a)-
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 108 of 154 PageID #: 991
109
P, Myeloperoxidase (MPO), Lp-PLA2, hs-CRP, Fibrinogen, Galectin-3, NT-proBNP, ApoE
Genotype, CYP2C19, Factor V Leiden, Prothrombin Mutation, MTHFR, Insulin, FFA (NEFA),
Uric Acid, Glucose, Hemoglobin A1C, Homocysteine, Thyroid Panel, Vitamin D, Cystatin-C,
Omega-3 & Omega-6 fatty acid profile, and Sterols. THD and LRH offered a “Little River Follow-
Up Panel” with the same tests. Grottenthaler, Madison, and Borgfeld approved the panels, and
Kash, Love, and LRH’s MSO recruiters arranged for and recommended that HCPs order the panels
or variations thereof.
495. THD and Stamford offered a “Stamford Assessment Panel” and “Stamford Follow-
Up Panel” that included the same tests as the Little River Assessment Panel. Grottenthaler
approved the panels, and Kash, Love, and Stamford’s MSO recruiters arranged for and
recommended that in routine clinical practice HCPs order the panels or variations thereof.
496. The default panel offered by BHD and LRH included over 30 specialty tests,
including HDL Map, Cholesterol Balance, Fatty Acid Balance Test, Apolipoprotein A1,
Apolipoprotein B, Total Cholesterol, Direct LDL-Cholesterol, HDL-Cholesterol, Lipoprotein a,
sdLDL-Cholesterol, Triglycerides, Albumin, Alkaline Phosphatase, ALT (SGPT), AST (SGOT),
BUN, Creatine Kinase, Creatinine, Homocysteine, NT-proBNP, Uric Acid, Vitamin D, Thyroid
Stimulating Hormone, hs-CRP, LpPLA2, Myeloperoxidase (MPO), Adiponectin, Glucose,
Hemoglobin A1c, Insulin, HOMA-IR, and CYP2C19 Genotype (Plavix). BHD and LRH described
the panel as “Panel M” for Medicare and other federal referrals. Hertzberg and Theiler approved
the panel, and Howard, Gonzales, Hardaway, Parnell, and LRH’s MSO recruiters arranged for and
recommended that in routine clinical practice HCPs order this panel or variations thereof.
497. The default panel offered by BHD and Stamford included all, or nearly all, of the
tests in LRH Panel M. Theiler approved the panel, and Howard, Gonzales, Hardaway, Parnell, and
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 109 of 154 PageID #: 992
110
LRH’s MSO recruiters arranged for and recommended that in routine clinical practice HCPs order
this panel or variations thereof.
498. Many of the individual tests within the panels marketed by defendants are
reasonable and necessary, if at all, only for specific patient populations with particular clinical
conditions. These tests are not reasonable and necessary in routine clinical practice for screening
an unselected or heterogenous population of patients in a PCP’s office. For example:
a. The Factor V Leiden test (CPT 81241) and Prothrombin Mutation test (CPT 81240)
each can detect a genetic defect that predisposes individuals to developing blood
clots. These tests are meant for patients who suffer blood clots within the veins
without any other predisposing cause for having one of these conditions.
b. Myeloperoxidase (MPO) is an enzyme found in white blood cells that is associated
with inflammation, and the MPO test (CPT 83876) is meant for patients presenting
with chest pain, the overwhelming majority of whom do not present to a PCP’s
office.
c. The CYP2C19 test (CPT 81225) is meant for those patients receiving Plavix
(clopidogrel) following coronary artery stenting.
d. The NT-proBNP test (CPT 83880) is meant for patients with dyspnea (shortness of
breath) to help make the diagnosis of congestive heart failure or to assess
cardiovascular risk in patients with acute coronary syndrome or stable coronary
artery disease.
e. The Galectin-3 test (CPT 82777) is meant to be used in conjunction with clinical
evaluation as an aid in assessing the prognosis of patients diagnosed with chronic
heart failure.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 110 of 154 PageID #: 993

111
499. Defendants arranged for and recommended that HCPs routinely order their
laboratory testing, without regard to specific patient needs.
500. By marketing and encouraging HCPs to order entire panels, which contained
individual laboratory tests that were inapplicable to many, if not most, patients, defendants
promoted the ordering of medically unnecessary laboratory testing.
501. Aware that routine ordering of the tests in their panels was unnecessary, defendants
advised HCPs on the diagnosis codes to use for certain tests, in order for the tests to be reimbursed
by insurers, including federal insurers. THD, BHD, and LRH even included sample diagnosis
codes in their laboratory test referral forms.
COUNT I
(Against All Defendants Except BenefitPro)
False Claims Act, 31 U.S.C. § 3729(a)(1)(A)
Presenting or Causing False Claims to Be Presented for Payment
502. The United States incorporates the preceding paragraphs here.
503. During the period of January 1, 2015 to December 31, 2017, all defendants except
BenefitPro knowingly submitted and/or caused LRH to submit the following four categories of
claims for payment to Medicare, Medicaid, and TRICARE for laboratory testing that were false or
fraudulent, and not payable.
504. First, all defendants except BenefitPro knowingly submitted and/or caused LRH to
submit to Medicare, Medicaid, and TRICARE claims for laboratory testing that were false or
fraudulent, and not payable, because of kickbacks that MSOs, including North Houston MSO,
Tomball MSO, Next Level MSOs, Quick MSO, Ascend MSO, and Rise MSOs, knowingly and
willfully paid to HCPs to induce their referrals to LRH, in violation of the AKS.
505. Second, all defendants except BenefitPro knowingly submitted and/or caused LRH
to submit to Medicare claims for laboratory testing that were improperly referred by physicians
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 111 of 154 PageID #: 994
112
whose financial relationships with LRH did not satisfy any applicable exception to the Stark Law.
LRH had an indirect compensation arrangement with the referring physicians who were receiving
MSO payments because there was an unbroken chain of persons with financial relationships
between them: LRH paid the recruiters, including S&G, Jacobs Marketing, Next Level, Exit
Therapy, APC, and LGRB; the recruiters paid the associated MSOs, including North Houston
MSO, Tomball MSO, Next Level MSOs, Quick MSO, Ascend MSO, and Rise MSOs; and the
MSOs paid the referring physicians.
506. The referring physicians received aggregate payments from the MSOs that varied
with or took into account the volume or value of referring physicians’ referrals to LRH for clinical
laboratory testing or other business generated by the referring physicians for LRH. LRH and each
defendant except BenefitPro knew that LRH was paying recruiters, who were directly or indirectly
providing MSO payments to LRH’s referring physicians. LRH and each defendant except
BenefitPro knew the referring physicians received aggregate payments from the MSOs that varied
with or took into account the volume or value of referring physicians’ referrals to LRH for clinical
laboratory testing or other business generated by the referring physicians for LRH. The financial
relationships between LRH and referring physicians did not satisfy the requirements of any
applicable exception to the Stark Law. The referring physicians referred Medicare beneficiaries to
LRH for clinical laboratory services, and LRH submitted claims to Medicare for those services.
Those physicians’ referrals to LRH for laboratory tests were prohibited, and the submission of the
claims for the improperly referred DHS to Medicare violated the Stark Law.
507. Third, all defendants except BenefitPro knowingly submitted and/or caused LRH
to submit to Medicare, Medicaid, and TRICARE claims for laboratory testing services for LRH
outpatients, despite knowing that the beneficiaries actually were non-patients of LRH.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 112 of 154 PageID #: 995

113
508. Fourth, all defendants except BenefitPro knowingly submitted and/or caused LRH
to submit to Medicare, Medicaid, and TRICARE claims for laboratory testing services that were
not reasonable and necessary for the diagnosis or treatment of an illness or injury or to improve
the functioning of a malformed body part.
509. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT II
(Against All Defendants Except BenefitPro)
False Claims Act, 31 U.S.C. § 3729(a)(1)(B)
Making or Using False Records or Statements
510. The United States incorporates the preceding paragraphs here.
511. During the period of January 1, 2015 to December 31, 2017, all defendants except
BenefitPro knowingly made or used, or caused to be made or used, false records or statements
material to false or fraudulent claims submitted to the United States, and payment of those false or
fraudulent claims by the United States was a reasonable and foreseeable consequence of those
defendants’ statements and actions.
512. These false records and statements included false certifications on provider
enrollment forms and false and misleading representations on claim forms, cost reports, and/or
hospital registration records that (1) LRH’s claims to Medicare, Medicaid, and TRICARE for
laboratory testing complied with the AKS, when in fact those claims violated the AKS; (2) LRH’s
claims to Medicare for laboratory testing complied with the Stark Law, when in fact those claims
violated the Stark Law; (3) LRH’s claims to Medicare, Medicaid, and TRICARE for laboratory
testing were for outpatients of LRH, when in fact those claims were for non-patients of LRH; and
(4) LRH’s claims to Medicare, Medicaid, and TRICARE for laboratory testing were reasonable
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 113 of 154 PageID #: 996

114
and necessary for the diagnosis or treatment of an illness or injury or to improve the functioning
of a malformed body part, when in fact those claims were not reasonable and necessary.
513. All defendants except BenefitPro made or used, or caused to be made or used, such
false records or statements with actual knowledge of their falsity, or with reckless disregard or
deliberate ignorance of whether or not they were false.
514. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT III
(Against Hertzberg, Theiler, Hickman, Howard, Gonzales, Madison, Borgfeld, Jones,
Parnell, Hardaway, Marioni, Perkins, Ginny Jacobs, Scott Jacobs, APM, APC, Next Level,
LGRB, S&G, and Jacobs Marketing)
False Claims Act, 31 U.S.C. § 3729(a)(1)(C)
Conspiracy to Submit False Claims
515. The United States incorporates the preceding paragraphs here.
516. Hertzberg, Theiler, Hickman, Howard, Gonzales, Madison, Borgfeld, Jones,
Parnell, Hardaway, Marioni, Perkins, Ginny Jacobs, Scott Jacobs, APM, APC, Next Level, LGRB,
S&G, and Jacobs Marketing knowingly entered into an unlawful agreement among themselves
and one or more others, including LRH and HCPs, to cause LRH to present false or fraudulent
claims to the United States, and performed acts in furtherance of this conspiracy. Specifically,
those defendants agreed to a plan by which, among other things, LRH paid recruiters, and funded
MSO kickbacks, to generate referrals to LRH for laboratory testing; LRH paid phlebotomists
located in HCPs’ offices to draw the beneficiaries’ blood; the phlebotomists were directed to create
false hospital registration records identifying the beneficiaries, who were non-patients of LRH, as
outpatients of LRH; the recruiters paid MSO kickbacks to HCPs to induce their referrals to LRH
for large panels of laboratory tests, regardless of whether the tests were reasonable and necessary;
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 114 of 154 PageID #: 997
115
BHD directly or indirectly performed the laboratory testing; and LRH submitted the resulting
claims for laboratory testing to Medicare, Medicaid, and TRICARE.
517. Defendants Madison and Borgfeld performed acts in furtherance of this conspiracy
by, among other things, entering into agreements with MSO recruiters to arrange for and
recommend referrals; entering into an agreement with BHD to perform laboratory testing for LRH;
calculating and/or authorizing payments from LRH to MSO recruiters to fund the MSO kickbacks;
calculating and/or authorizing payments from LRH to BHD for performing laboratory testing;
authorizing agreements with phlebotomists who worked in referring HCPs’ offices; reviewing
and/or submitting claims to Medicare, Medicaid, and TRICARE; and reviewing and/or signing
Medicare cost reports.
518. Defendants Ginny Jacobs, Scott Jacobs, S&G, and Jacobs Marketing performed
acts in furtherance of this conspiracy by, among other things, entering into agreements with LRH
to arrange for and recommend referrals; recruiting HCPs to refer to LRH by offering them MSO
kickbacks; transferring funds from S&G and Jacobs Marketing by means of direct and indirect
transfers to North Houston MSO and Tomball MSO; and paying kickbacks to HCPs through North
Houston MSO and Tomball MSO.
519. Defendants Howard and Gonzales performed acts in furtherance of this conspiracy
by, among other things, attending in person meetings with potential HCP referral sources;
recruiting HCPs to refer to LRH by offering them kickbacks from Quick MSO and Ascend MSO;
providing information and/or documentation to HCPs about the MSO kickbacks; receiving
documentation from HCPs; providing and/or coordinating the delivery of MSO checks to HCPs;
directing BHD personnel to provide supplies and shipping materials to HCPs; meeting with LRH
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 115 of 154 PageID #: 998
116
personnel about the laboratory referrals; and receiving money, directly or indirectly, as
compensation for recruiting HCPs to refer to LRH.
520. Defendant Hickman performed acts in furtherance of this conspiracy by, among
other things, creating, owning, and operating APM, APC, and the Ascend MSO; meeting with at
least one HCP about kickbacks from Ascend MSO; depositing purported investment checks that
HCPs provided to Ascend MSO; authorizing and signing purported distribution checks from
Ascend MSO to HCPs; authorizing and signing checks and/or bank transfers to himself and
Zalegon for Gonzales’ and Howard’s benefit.
521. Defendants Stanley Jones, Jeffrey Parnell, Thomas Gray Hardaway, and LGRB
performed acts in furtherance of this conspiracy by, among other things, entering into agreements
with LRH to arrange for and recommend referrals; providing information and/or documentation
to HCPs about the MSO kickbacks; receiving documentation from HCPs; providing and/or
coordinating the delivery of MSO checks to HCPs; recruiting HCPs to refer to LRH by offering
them MSO kickbacks; transferring funds from LGRB to Rise MSOs; authorizing and signing
checks and/or bank transfers to Jones, Parnell, and Hardaway as compensation for recruiting HCPs
to refer to LRH; and paying kickbacks to HCPs through Rise MSOs.
522. Defendants Ruben Marioni, Jordan Perkins, and Next Health performed acts in
furtherance of this conspiracy by, among other things, entering into agreements with LRH to
arrange for and recommend referrals; providing information and/or documentation to HCPs about
the MSO kickbacks; receiving documentation from HCPs; providing and/or coordinating the
delivery of MSO checks to HCPs; recruiting HCPs to refer to LRH by offering them MSO
kickbacks; transferring funds from Next Level to Next Level MSOs; authorizing and signing
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 116 of 154 PageID #: 999

117
checks and/or bank transfers to Marioni and Perkins as compensation for recruiting HCPs to refer
to LRH; and paying kickbacks to HCPs through Next Level MSOs.
523. Defendants Hertzberg and Theiler performed acts in furtherance of this conspiracy
by, among other things, entering into an agreement with LRH to provide laboratory testing;
meeting in person or by remote means with MSO recruiters, LRH personnel, and HCPs; meeting
with BHD personnel about the MSO kickbacks and laboratory referrals to LRH; and authorizing
and paying commissions and bonuses to BHD sales personnel based on HCPs’ referrals to LRH
for BHD testing.
524. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT IV
(Against Grottenthaler, Kash, Cornwell, Love, Hickman, Gonzales, Madison, Borgfeld,
Jones, Parnell, Hardaway, Marioni, Perkins, Ginny Jacobs, Scott Jacobs, APM, APC, Next
Level, LGRB, S&G, and Jacobs Marketing)
False Claims Act, 31 U.S.C. § 3729(a)(1)(C)
Conspiracy to Submit False Claims
525. The United States incorporates the preceding paragraphs here.
526. Grottenthaler, Kash, Cornwell, Love, Hickman, Gonzales, Madison, Borgfeld,
Jones, Parnell, Hardaway, Marioni, Perkins, Ginny Jacobs, Scott Jacobs, APM, APC, Next Level,
LGRB, S&G, and Jacobs Marketing knowingly entered into an unlawful agreement among
themselves and one or more others, including LRH and HCPs, to cause LRH to present false or
fraudulent claims to the United States, and performed acts in furtherance of this conspiracy.
Specifically, those defendants agreed to a plan by which, among other things, LRH paid recruiters,
and funded MSO kickbacks, to generate referrals to LRH for laboratory testing; LRH paid
phlebotomists located in HCPs’ offices to draw the beneficiaries’ blood; the phlebotomists were
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 117 of 154 PageID #: 1000
118
directed to create false hospital registration records identifying the beneficiaries, who were non-
patients of LRH, as outpatients of LRH; the recruiters paid MSO kickbacks to HCPs to induce
their referrals to LRH for large panels of laboratory tests, regardless of whether the tests were
reasonable and necessary; THD directly or indirectly performed the laboratory testing; and LRH
submitted the resulting claims for laboratory testing to Medicare, Medicaid, and TRICARE.
527. Defendants Madison and Borgfeld performed acts in furtherance of this conspiracy
by, among other things, entering into agreements with MSO recruiters to arrange for and
recommend referrals; entering into an agreement with THD to perform laboratory testing for LRH;
calculating and/or authorizing payments from LRH to MSO recruiters to fund the MSO kickbacks;
calculating and/or authorizing payments from LRH to THD for performing laboratory testing;
authorizing agreements with phlebotomists who worked in referring HCPs’ offices; reviewing
and/or submitting claims to Medicare, Medicaid, and TRICARE; and reviewing and/or signing
Medicare cost reports.
528. Defendants Ginny Jacobs, Scott Jacobs, S&G, and Jacobs Marketing performed
acts in furtherance of this conspiracy by, among other things, entering into agreements with LRH
to arrange for and recommend referrals; recruiting HCPs to refer to LRH by offering them MSO
kickbacks; transferring funds from S&G and Jacobs Marketing by means of direct and indirect
transfers to North Houston MSO and Tomball MSO; and paying kickbacks to HCPs through North
Houston MSO and Tomball MSO.
529. Defendants Kash and Gonzales performed acts in furtherance of this conspiracy by,
among other things, attending in person meetings with potential HCP referral sources; recruiting
HCPs to refer to LRH by offering them kickbacks from Quick MSO and/or Ascend MSO;
providing information and/or documentation to HCPs about the MSO kickbacks; receiving
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 118 of 154 PageID #: 1001
119
documentation from HCPs; providing and/or coordinating the delivery of MSO checks to HCPs;
directing THD personnel to provide supplies and shipping materials to HCPs; meeting with LRH
personnel about the laboratory referrals; and receiving money, directly or indirectly, as
compensation for recruiting HCPs to refer to LRH.
530. Defendant Hickman performed acts in furtherance of this conspiracy by, among
other things, creating, owning, and operating APM, APC, and the Ascend MSO; meeting with at
least one HCP about kickbacks from Ascend MSO; depositing purported investment checks that
HCPs provided to Ascend MSO; authorizing and signing purported distribution checks from
Ascend MSO to HCPs; authorizing and signing checks and/or bank transfers to himself, Zalegon
for Gonzales’ benefit, and Tigerlily for Kash’s benefit.
531. Defendants Stanley Jones, Jeffrey Parnell, Thomas Gray Hardaway, and LGRB
performed acts in furtherance of this conspiracy by, among other things, entering into agreements
with LRH to arrange for and recommend referrals; providing information and/or documentation
to HCPs about the MSO kickbacks; receiving documentation from HCPs; providing and/or
coordinating the delivery of MSO checks to HCPs; recruiting HCPs to refer to LRH by offering
them MSO kickbacks; transferring funds from LGRB to Rise MSOs; authorizing and signing
checks and/or bank transfers to Jones, Parnell, and Hardaway as compensation for recruiting HCPs
to refer to LRH; and paying kickbacks to HCPs through Rise MSOs.
532. Defendants Ruben Marioni, Jordan Perkins, and Next Health performed acts in
furtherance of this conspiracy by, among other things, entering into agreements with LRH to
arrange for and recommend referrals; providing information and/or documentation to HCPs about
the MSO kickbacks; receiving documentation from HCPs; providing and/or coordinating the
delivery of MSO checks to HCPs; recruiting HCPs to refer to LRH by offering them MSO
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 119 of 154 PageID #: 1002
120
kickbacks; transferring funds from Next Level to Next Level MSOs; authorizing and signing
checks and/or bank transfers to Marioni and Perkins as compensation for recruiting HCPs to refer
to LRH; and paying kickbacks to HCPs through Next Level MSOs.
533. Defendant Grottenthaler performed acts in furtherance of this conspiracy by,
among other things, entering into agreements with LRH to provide laboratory testing, consulting,
equipment leases, and billing services; meeting in person with MSO recruiters, LRH personnel,
and HCPs; hiring THD personnel to implement the LRH fraud scheme; meeting with THD
personnel about the MSO kickbacks and laboratory referrals to LRH; and authorizing and paying
commissions and bonuses to THD sales personnel based on HCPs’ referrals to LRH for THD
testing.
534. Defendants Cornwell, Kash, and Love performed acts in furtherance of this
conspiracy by, among other things, attending in person meetings with potential HCP referral
sources; meeting with MSO recruiters; recruiting HCPs to refer to LRH by offering them, and/or
referring them to MSO recruiters to receive, MSO kickbacks; providing information and/or
documentation to HCPs about LRH and THD testing and the MSO kickbacks; directing THD
personnel to provide supplies and shipping materials to HCPs; meeting with LRH personnel about
the laboratory referrals; and receiving commissions from THD based on HCPs’ referrals to LRH
for THD testing.
535. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 120 of 154 PageID #: 1003

121
COUNT V
(Against Grottenthaler, Theiler, Kash, Howard, Gonzales, Love, Hickman, Marioni,
Perkins, APM, and BenefitPro)
False Claims Act, 31 U.S.C. § 3729(a)(1)(A)
Presenting or Causing False Claims to Be Presented for Payment
536. The United States incorporates the preceding paragraphs here.
537. During the period of May 1, 2017 to May 31, 2018, Defendants Grottenthaler,
Theiler, Kash, Howard, Gonzales, Love, Hickman, Marioni, Perkins, APM, and BenefitPro
knowingly caused THD and/or BHD to submit claims for payment to Medicare, Medicaid, and
TRICARE for laboratory testing that were false or fraudulent, and not payable, because of the
kickbacks paid from BenefitPro MSOs and Regal MSOs to HCPs to induce their referrals to THD
and/or BHD for laboratory testing.
538. In addition, defendants Grottenthaler, Theiler, Kash, Howard, Gonzales, Love,
Hickman, APM, and BenefitPro knowingly submitted and/or caused THD and/or BHD to submit
to Medicare, Medicaid, and TRICARE claims for laboratory testing services that were not
reasonable and necessary for the diagnosis or treatment of an illness or injury or to improve the
functioning of a malformed body part.
539. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT VI
(Against Grottenthaler, Theiler, Kash, Howard, Gonzales, Love, Hickman, Marioni,
Perkins, APM, and BenefitPro)
False Claims Act, 31 U.S.C. § 3729(a)(1)(B)
Making or Using False Records or Statements
540. The United States incorporates the preceding paragraphs here.
541. During the period of May 1, 2017 to May 31, 2018, defendants Grottenthaler,
Theiler, Kash, Howard, Gonzales, Love, Hickman, Marioni, Perkins, APM, and BenefitPro
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 121 of 154 PageID #: 1004

122
knowingly made or used, or caused to be made or used, false records or statements material to
false or fraudulent claims submitted to the United States, and payment of those false or fraudulent
claims by the United States was a reasonable and foreseeable consequence of defendants’
statements and actions.
542. The false records and statements included false certifications on provider
enrollment forms and false and misleading representations on claim forms that THD and/or BHD’s
claims to Medicare, Medicaid, and TRICARE for laboratory testing (1) complied with the AKS,
when in fact those claims violated the AKS; and/or (2) were reasonable and necessary for the
diagnosis or treatment of an illness or injury or to improve the functioning of a malformed body
part, when in fact those claims were not reasonable and necessary.
543. Defendants Grottenthaler, Theiler, Kash, Howard, Gonzales, Love, Hickman,
Marioni, Perkins, APM, and BenefitPro made or used, or caused to be made or used, such false
records or statements with actual knowledge of their falsity, or with reckless disregard or deliberate
ignorance of whether or not they were false.
544. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT VII
(Against Grottenthaler, Kash, Gonzales, Love, Hickman, Marioni, Perkins, APM, and
BenefitPro)
False Claims Act, 31 U.S.C. § 3729(a)(1)(C)
Conspiracy to Submit False Claims
545. The United States incorporates the preceding paragraphs here.
546. Defendants Grottenthaler, Kash, Gonzales, Love, Hickman, Marioni, Perkins,
APM, and BenefitPro knowingly entered into an unlawful agreement among themselves and with
one or more others, including Stamford and HCPs, to submit or cause THD to submit false or
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 122 of 154 PageID #: 1005
123
fraudulent claims to the United States, and performed acts in furtherance of this conspiracy.
Specifically, those defendants agreed to a plan by which, among other things, MSO recruiters
would pay MSO kickbacks to HCPs to induce their referrals to THD for laboratory testing, THD
would perform the laboratory testing, and THD would submit the claims to Medicare, Medicaid,
and TRICARE.
547. Defendant Hickman performed acts in furtherance of this conspiracy by, among
other things, creating, owning, and operating APM, BenefitPro, and the BenefitPro MSO;
depositing purported investment checks that HCPs provided to BenefitPro MSOs; authorizing and
signing purported distribution checks from BenefitPro MSOs to HCPs; authorizing and signing
checks and/or bank transfers to himself, Regal, Zalegon for Gonzales’ benefit, and Tigerlily for
Kash’s benefit.
548. Defendants Kash and Gonzales performed acts in furtherance of this conspiracy by,
among other things, attending in person meetings with potential HCP referral sources; recruiting
HCPs to refer to THD by offering them kickbacks from BenefitPro MSOs; providing information
and/or documentation to HCPs about the MSO kickbacks; receiving documentation from HCPs;
providing and/or coordinating the delivery of MSO checks to HCPs; directing THD personnel to
provide supplies, requisition forms, and shipping materials to HCPs; meeting with THD personnel
about the laboratory referrals; and receiving money, directly or indirectly, as compensation for
recruiting HCPs to refer to THD.
549. Defendants Marioni and Perkins performed acts in furtherance of this conspiracy
by, among other things, entering into an agreement with BenefitPro on behalf of Regal; recruiting
HCPs to refer to THD by offering them kickbacks from Regal MSOs; providing information and/or
documentation to HCPs about the MSO kickbacks; receiving documentation from HCPs;
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 123 of 154 PageID #: 1006

124
providing and/or coordinating the delivery of MSO checks to HCPs; directing THD personnel to
provide supplies, requisition forms, and shipping materials to HCPs; meeting with THD personnel
about the laboratory referrals; and receiving money, directly or indirectly, as compensation for
recruiting HCPs to refer to THD.
550. Defendant Grottenthaler performed acts in furtherance of this conspiracy by,
among other things, meeting in person with MSO recruiters, Stamford personnel, and HCPs;
meeting with THD personnel about the MSO kickbacks and laboratory referrals to THD; and
authorizing and paying commissions and bonuses to THD sales personnel based on HCPs’ referrals
for THD testing.
551. Defendants Kash and Love performed acts in furtherance of this conspiracy by,
among other things, attending in person meetings with potential HCP referral sources; meeting
with MSO recruiters; recruiting HCPs to refer to THD by offering them, and/or referring them to
MSO recruiters to receive, MSO kickbacks; providing information and/or documentation to HCPs
about Stamford and THD testing and the MSO kickbacks; directing THD personnel to provide
supplies, requisition forms, and shipping materials to HCPs; meeting with Stamford personnel
about the laboratory referrals; and receiving commissions from THD based on HCPs’ referrals to
THD for laboratory testing.
552. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT VIII
(Against Theiler, Howard, Gonzales, Hickman, Marioni, Perkins, APM, and BenefitPro)
False Claims Act, 31 U.S.C. § 3729(a)(1)(C)
Conspiracy to Submit False Claims
553. The United States incorporates the preceding paragraphs here.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 124 of 154 PageID #: 1007
125
554. Defendants Theiler, Howard, Gonzales, Hickman, Marioni, Perkins, APM, and
BenefitPro knowingly entered into an unlawful agreement among themselves and with one or more
others, including Stamford and HCPs, to cause BHD to present false or fraudulent claims to the
United States, and performed acts in furtherance of this conspiracy. Specifically, those defendants
agreed to a plan by which, among other things, MSO recruiters would pay MSO kickbacks to
HCPs to induce their referrals to BHD for laboratory testing, BHD would perform the laboratory
testing, and BHD would submit the claims to Medicare, Medicaid, and TRICARE.
555. Defendant Hickman performed acts in furtherance of this conspiracy by, among
other things, creating, owning, and operating APM, BenefitPro, and the BenefitPro MSO;
depositing purported investment checks that HCPs provided to BenefitPro MSOs; authorizing and
signing purported distribution checks from BenefitPro MSOs to HCPs; authorizing and signing
checks and/or bank transfers to himself, Regal, and Zalegon for Gonzales’ and Howard’s benefit.
556. Defendants Howard and Gonzales performed acts in furtherance of this conspiracy
by, among other things, attending in person meetings with potential HCP referral sources;
recruiting HCPs to refer to BHD by offering them kickbacks from BenefitPro MSOs; providing
information and/or documentation to HCPs about the MSO kickbacks; receiving documentation
from HCPs; providing and/or coordinating the delivery of MSO checks to HCPs; directing BHD
personnel to provide supplies, requisition forms, and shipping materials to HCPs; meeting with
BHD personnel about the laboratory referrals; and receiving money, directly or indirectly, as
compensation for recruiting HCPs to refer to BHD.
557. Defendants Marioni and Perkins performed acts in furtherance of this conspiracy
by, among other things, entering into an agreement with BenefitPro on behalf of Regal; recruiting
HCPs to refer to BHD by offering them kickbacks from Regal MSOs; providing information
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 125 of 154 PageID #: 1008

126
and/or documentation to HCPs about the MSO kickbacks; receiving documentation from HCPs;
providing and/or coordinating the delivery of MSO checks to HCPs; directing BHD personnel to
provide supplies, requisition forms, and shipping materials to HCPs; meeting with BHD personnel
about the laboratory referrals; and receiving money, directly or indirectly, as compensation for
recruiting HCPs to refer to BHD.
558. Defendant Theiler performed acts in furtherance of this conspiracy by, among other
things, meeting with MSO recruiters, Stamford personnel, and HCPs; meeting with BHD
personnel about the MSO kickbacks and laboratory referrals to BHD; and receiving commissions
and/or bonuses from BHD based on HCPs’ referrals to BHD for laboratory testing.
559. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT IX
(Against Grottenthaler, Hertzberg, Theiler, Cornwell, and Love)
False Claims Act, 31 U.S.C. § 3729(a)(1)(A)
Presenting or Causing False Claims to Be Presented for Payment
560. The United States incorporates the preceding paragraphs here.
561. During the period of July 1, 2015 to December 31, 2016, Defendants Grottenthaler,
Hertzberg, Theiler, Cornwell, and Love knowingly caused THD and/or BHD to submit claims for
payment to Medicare, Medicaid, and TRICARE for laboratory testing that were false or fraudulent,
and not payable, because of the kickbacks paid from ITH MSOs, including Vybrem and
Benchmark, to HCPs to induce their referrals to THD and/or BHD for laboratory testing.
562. In addition, defendants Grottenthaler, Hertzberg, Theiler, Cornwell, and Love
knowingly submitted and/or caused THD and/or BHD to submit to Medicare, Medicaid, and
TRICARE claims for laboratory testing services that were not reasonable and necessary for the
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 126 of 154 PageID #: 1009

127
diagnosis or treatment of an illness or injury or to improve the functioning of a malformed body
part.
563. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT X
(Against Grottenthaler, Hertzberg, Theiler, Cornwell, and Love)
False Claims Act, 31 U.S.C. § 3729(a)(1)(B)
Making or Using False Records or Statements
564. The United States incorporates the preceding paragraphs here.
565. During the period of July 1, 2015 to December 31, 2016, Defendants Grottenthaler,
Hertzberg, Theiler, Cornwell, and Love knowingly made or used, or caused to be made or used,
false records or statements material to false or fraudulent claims submitted to the United States,
and payment of those false or fraudulent claims by the United States was a reasonable and
foreseeable consequence of defendants’ statements and actions.
566. The false records and statements included false certifications on provider
enrollment forms and false and misleading representations on claim forms that THD and/or BHD’s
claims to Medicare, Medicaid, and TRICARE for laboratory testing (1) complied with the AKS,
when in fact those claims violated the AKS because of the kickbacks paid from ITH MSOs,
including Vybrem and Benchmark, to HCPs to induce their referrals to THD and/or BHD for
laboratory testing; and/or (2) were reasonable and necessary for the diagnosis or treatment of an
illness or injury or to improve the functioning of a malformed body part, when in fact those claims
were not reasonable and necessary.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 127 of 154 PageID #: 1010

128
567. Defendants Grottenthaler, Hertzberg, Theiler, Cornwell, and Love made or used,
or caused to be made or used, such false records or statements with actual knowledge of their
falsity, or with reckless disregard or deliberate ignorance of whether or not they were false.
568. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT XI
(Against Grottenthaler, Cornwell, and Love)
False Claims Act, 31 U.S.C. § 3729(a)(1)(C)
Conspiracy to Submit False Claims
569. The United States incorporates the preceding paragraphs here.
570. Defendants Grottenthaler, Cornwell, and Love knowingly entered into an unlawful
agreement among themselves and with one or more others, including ITH and HCPs, to submit or
cause THD to submit false or fraudulent claims to the United States, and performed acts in
furtherance of this conspiracy. Specifically, those defendants agreed to a plan by which, among
other things, MSO recruiters would pay MSO kickbacks to HCPs to induce their referrals to THD
for laboratory testing, THD would perform the laboratory testing, and THD would submit the
claims to Medicare, Medicaid, and TRICARE.
571. Defendant Grottenthaler performed acts in furtherance of this conspiracy by,
among other things, meeting in person with MSO recruiters, ITH personnel, and HCPs; meeting
with THD personnel about the MSO kickbacks and laboratory referrals to THD; and authorizing
and paying commissions and bonuses to THD sales personnel based on HCPs’ referrals for THD
testing.
572. Defendants Cornwell and Love performed acts in furtherance of this conspiracy by,
among other things, attending in person meetings with potential HCP referral sources; meeting
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 128 of 154 PageID #: 1011

129
with MSO recruiters; recruiting HCPs to refer to THD by offering them, and/or referring them to
MSO recruiters to receive, MSO kickbacks; providing information and/or documentation to HCPs
about ITH and THD testing and the MSO kickbacks; directing THD personnel to provide supplies,
requisition forms, and shipping materials to HCPs; meeting with ITH personnel about the
laboratory referrals; and receiving commissions from THD based on HCPs’ referrals to THD for
laboratory testing.
573. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT XII
(Against Hertzberg and Theiler)
False Claims Act, 31 U.S.C. § 3729(a)(1)(C)
Conspiracy to Submit False Claims
574. The United States incorporates the preceding paragraphs here.
575. Defendants Hertzberg and Theiler knowingly entered into an unlawful agreement
among themselves and with one or more others, including ITH and HCPs, to submit or cause BHD
to submit false or fraudulent claims to the United States, and performed acts in furtherance of this
conspiracy. Specifically, those defendants agreed to a plan by which, among other things, MSO
recruiters would pay MSO kickbacks to HCPs to induce their referrals to BHD for laboratory
testing, THD would perform the laboratory testing, and BHD would submit the claims to Medicare,
Medicaid, and TRICARE.
576. Defendant Hertzberg performed acts in furtherance of this conspiracy by, among
other things, authorizing BHD’s arrangement with ITH; communicating with BHD personnel
about ITH and the MSO kickbacks and laboratory referrals to BHD; and authorizing and paying
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 129 of 154 PageID #: 1012

130
commissions and/or bonuses to BHD sales personnel based on HCPs’ referrals to BHD for
laboratory testing.
577. Defendant Theiler performed acts in furtherance of this conspiracy by, among other
things, authorizing BHD’s arrangement with ITH; communicating with MSO recruiters, ITH
personnel, and HCPs; communicating with BHD personnel about ITH and the MSO kickbacks
and laboratory referrals to BHD; and receiving commissions and/or bonuses from BHD based on
HCPs’ referrals to BHD for laboratory testing.
578. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT XIII
(Against Grottenthaler, Cornwell, and Love)
False Claims Act, 31 U.S.C. § 3729(a)(1)(A)
Presenting or Causing False Claims to Be Presented for Payment
579. The United States incorporates the preceding paragraphs here.
580. During the period of January 1, 2015 to May 31, 2018, Grottenthaler, Cornwell,
and Love knowingly submitted and/or caused THD to submit the following five categories of
claims for payment to Medicare, Medicaid, and TRICARE for laboratory testing that were false or
fraudulent, and not payable.
581. First, Grottenthaler, Cornwell, and Love knowingly submitted and/or caused THD
to submit to Medicare, Medicaid, and TRICARE claims for laboratory testing that were false or
fraudulent, and not payable, because of the P&H fee kickbacks that THD paid directly or indirectly
to HCPs to induce their referrals to THD, in violation of the AKS.
582. Second, Grottenthaler, Cornwell, and Love knowingly submitted and/or caused
THD to submit to Medicare, Medicaid, and TRICARE claims for laboratory testing that were false
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 130 of 154 PageID #: 1013
131
or fraudulent, and not payable, because of the consulting fee kickbacks that THD paid to Physician
K to induce his referrals to THD, in violation of the AKS.
583. Third, Grottenthaler, Cornwell, and Love knowingly submitted and/or caused THD
to submit to Medicare, Medicaid, and TRICARE claims for laboratory testing that were false or
fraudulent, and not payable, because of the kickbacks in the form of waived patient copayments
and deductibles, in violation of the AKS.
584. Fourth, Grottenthaler, Cornwell, and Love knowingly submitted and/or caused
THD to submit to Medicare claims for laboratory testing that were improperly referred by
physicians with whom THD had a financial relationship that did not satisfy the requirements of an
applicable exception to the Stark Law. THD had a direct compensation arrangement with
Physician K in which THD paid Physician K purported consulting fees. In addition, THD had an
indirect compensation arrangement with the referring physicians who received P&H fee payments
through purported draw site companies, as THD paid the companies, and the companies paid
physicians and the physicians’ immediate family members. The referring physicians or their
immediate family members received P&H fee payments from THD that varied with or took into
account the volume or value of referring physicians’ referrals to THD for clinical laboratory testing
or other business generated by the referring physicians for THD. THD, Grottenthaler, Cornwell,
and Love knew that the physicians received aggregate compensation from THD and the companies
THD paid that varied with or otherwise took into account the volume or value of their referrals to
THD. The financial relationships between THD and referring physicians or the physicians’
immediate family members due to the P&H fee payments did not satisfy any Stark Law exception.
The referring physicians referred Medicare beneficiaries to THD for clinical laboratory services,
and THD submitted claims to Medicare for those services. Those physicians’ referrals to THD for
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 131 of 154 PageID #: 1014

132
laboratory tests were prohibited, and the submission of the claims for the improperly referred DHS
to Medicare violated the Stark Law.
585. Fifth, Grottenthaler, Cornwell, and Love knowingly submitted and/or caused THD
to submit to Medicare, Medicaid, and TRICARE claims for laboratory testing services that were
not reasonable and necessary for the diagnosis or treatment of an illness or injury or to improve
the functioning of a malformed body part.
586. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT XIV
(Against Grottenthaler, Cornwell, and Love)
False Claims Act, 31 U.S.C. § 3729(a)(1)(B)
Making or Using False Records or Statements
587. The United States incorporates the preceding paragraphs here.
588. During the period of January 1, 2015 to May 31, 2018, Grottenthaler, Cornwell,
and Love knowingly made or used, or caused to be made or used, false records or statements
material to false or fraudulent claims submitted to the United States, and payment of those false or
fraudulent claims by the United States was a reasonable and foreseeable consequence of those
defendants’ statements and actions.
589. These false records and statements included false certifications on provider
enrollment forms and false and misleading representations on claim forms that (1) THD’s claims
to Medicare, Medicaid, and TRICARE for laboratory testing complied with the AKS, when in fact
those claims violated the AKS; (2) THD’s claims to Medicare for laboratory testing complied with
the Stark Law, when in fact those claims violated the Stark Law; and (3) THD’s claims to
Medicare, Medicaid, and TRICARE for laboratory testing were reasonable and necessary for the
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 132 of 154 PageID #: 1015

133
diagnosis or treatment of an illness or injury or to improve the functioning of a malformed body
part, when in fact those claims were not reasonable and necessary.
590. In addition, the false records and statements included false and misleading
statements and representations, including on THD payment documents, that THD paid consulting
fees to Physician K for participating in THD’s advisory board, when in fact no such board existed
at THD; and that THD paid P&H fees to draw site companies that were independent of referring
HCPs, when in fact the draw site companies were conduits to pay P&H fees directly or indirectly
to HCPs to induce referrals for laboratory testing.
591. Grottenthaler, Cornwell, and Love made or used, or caused to be made or used,
such false records or statements with actual knowledge of their falsity, or with reckless disregard
or deliberate ignorance of whether or not they were false.
592. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT XV
(Against Grottenthaler, Cornwell, and Love)
False Claims Act, 31 U.S.C. § 3729(a)(1)(C)
Conspiracy to Submit False Claims
593. The United States incorporates the preceding paragraphs here.
594. Grottenthaler, Cornwell, and Love knowingly entered into an unlawful agreement
among themselves and with one or more others, including Sultan, Zabeda, Physician K, and other
HCPs, to cause the presentation of false or fraudulent claims to the United States, and performed
acts in furtherance of this conspiracy. Specifically, those defendants agreed to a plan by which
THD would submit claims to Medicare, Medicaid, and TRICARE for laboratory testing, where
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 133 of 154 PageID #: 1016

134
such claims violated the AKS and Stark Law and were for tests that were not reasonable and
necessary.
595. Grottenthaler performed acts in furtherance of this conspiracy by, among other
things, communicating with purported draw site companies, authorizing purported P&H fee and
consulting payments, approving and communicating THD’s copayment and deductible waiver
policy, and approving THD panels and requisition forms.
596. Cornwell and Love performed acts in furtherance of this conspiracy by, among
other things, communicating with purported draw site companies, offering purported P&H fee and
consulting payments to HCPs or immediate family members of HCPs, offering copayment and
deductible waivers to HCPs, and arranging for and recommending that HCPs order tests on THD’s
panels and requisition forms.
597. By virtue of these false or fraudulent claims, the United States suffered damages
and therefore is entitled to treble damages under the FCA, to be determined at trial, plus civil
penalties for each violation.
COUNT XVI
(Against All Defendants)
Unjust Enrichment
598. The United States incorporates the preceding paragraphs here.
599. This is a claim for the recovery of monies by which defendants have been unjustly
enriched.
600. By directly or indirectly obtaining from the United States, through Medicare,
Medicaid, and TRICARE, funds to which they were not entitled, defendants were unjustly
enriched, and are liable to account and pay such amounts, or the proceeds therefrom, which are to
be determined at trial.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 134 of 154 PageID #: 1017

135
COUNT XVII
(Against All Defendants)
Payment by Mistake
601. The United States incorporates the preceding paragraphs here.
602. This is a claim for the recovery of monies the United States paid directly or
indirectly to defendants as a result of mistaken understandings of fact.
603. The United States’ mistaken understandings of fact were material to its decision to
pay the claims to Medicare, Medicaid, and TRICARE that were submitted or caused to be
submitted by defendants for laboratory testing.
604. The United States, acting in reasonable reliance on the truthfulness of the claims
and the truthfulness of associated statements, certifications, and representations, paid monies
directly or indirectly to defendants to which they were not entitled. Thus, the United States is
entitled to recoup such monies, in an amount to be determined at trial.
PRAYER FOR RELIEF
The United States requests that judgment be entered in its favor and against the defendants
identified above as follows:
(a) On Counts I–XV (False Claims Act), for treble the United States’ damages,
together with the maximum civil penalties allowed by law;
(b) On Count XVI (Unjust Enrichment), in the amount by which defendants were
unjustly enriched;
(c) On Count XVII (Payment by Mistake), in the amount mistakenly paid to
defendants; and
(d) Pre- and post-judgment interest, costs, and such other relief as the Court may deem
appropriate.
JURY DEMAND
Pursuant to Federal Rule of Civil Procedure 38, the United States requests a trial by jury.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 135 of 154 PageID #: 1018

136
Date: January 31, 2022. Respectfully Submitted,
BRIAN M. BOYNTON
Acting Assistant Attorney General
Civil Division
BRIT FEATHERSTON
United States Attorney
/s/ James Gillingham_____________
JAMES GILLINGHAM
ADRIAN GARCIA
BETTY YOUNG
Assistant U.S. Attorneys
Eastern District of Texas
110 N. College Street; Suite 700
Tyler, Texas 75702
E-mail: [email protected]
E-mail: AGar[email protected]
E-mail: [email protected]
(903) 590-1400
(903) 590-1436 (fax)
Texas State Bar # 24065295
JAMIE ANN YAVELBERG
SARA MCLEAN
CHRISTOPHER TERRANOVA
Civil Division
U.S. Department of Justice
Post Office Box 261
Ben Franklin Station
Washington, DC 20044
(202) 616-4203
(202) 514-0280 (fax)
Attorneys for the United States
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 136 of 154 PageID #: 1019

137
EXHIBIT A
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
80061
Blood test, lipids (cholesterol and triglycerides)
$112.84
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 81225
Gene analysis (cytochrome P450, family 2,
subfamily C, polypeptide 19) common variants
$650.21
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 81240
Gene analysis (prothrombin, coagulation factor II)
A variant
$353.78
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 81241
Gene analysis (coagulation factor V) Leiden
variant
$242.80
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
81401
Molecular pathology procedure level 2
$207.80
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
82040
Albumin (protein) level
$51.02
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
82172
Apolipoprotein level
$94.59
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
82306
Vitamin D-3 level
$125.13
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
82550
Creatine kinase (cardiac enzyme) level, total
$75.22
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
82565
Blood creatinine level
$47.29
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 82627
Dehydroepiandrosterone (DHEA-S) hormone
level
$67.78
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
82670
Measurement of total estradiol (hormone)
$94.22
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
82947
Blood glucose (sugar) level
$59.96
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
83036
Hemoglobin A1C level
$46.92
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 83519
Measurement of substance using immunoassay
technique, by radioimmunoassay
$430.12
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 83520
Measurement of substance using immunoassay
technique
$92.73
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
83525
Insulin measurement, total
$46.92
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
83695
Lipoprotein (A) level
$39.47
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$103.15
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
83701
Lipoprotein measurement
$75.60
2
For patient privacy, their names have been omitted from this chart and replaced with a numerical identifier.
3
Date of the initial claim to a federal healthcare program; for LRH claims, see paragraph 289 above for the date the final claim was
submitted as part of LRH’s cost report.
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 137 of 154 PageID #: 1020

138
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$103.15
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 83880
Natriuretic peptide (heart and blood vessel
protein) level
$103.15
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
83921
Organic acid level
$121.40
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
84075
Phosphatase (enzyme) level, alkaline
$76.71
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
84270
Sex hormone binding globulin (protein) level
$65.91
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
84402
Testosterone (hormone) level, free
$24.95
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
84403
Testosterone (hormone) level, total
$101.67
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
84443
Blood test, thyroid stimulating hormone (TSH)
$64.43
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
84450
Liver enzyme (SGOT), level
$15.64
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
84460
Liver enzyme (SGPT), level
$51.39
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 84520
Urea nitrogen level to assess kidney function,
quantitative
$40.22
Physician A
LRH
Medicare
Beneficiary 1
4/15/2016
5/27/2016
84550
Uric acid level, blood
$54.00
Physician A LRH Medicare Beneficiary 1 4/15/2016 5/27/2016 86141
Measurement C-reactive protein for detection of
infection or inflammation, high sensitivity
$102.41
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
80061
Blood test, lipids (cholesterol and triglycerides)
$112.84
Physician A LRH Medicare Beneficiary 2 9/13/2016 10/26/2016 81225
Gene analysis (cytochrome P450, family 2,
subfamily C, polypeptide 19) common variants
$650.21
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
82040
Albumin (protein) level
$51.02
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
82172
Apolipoprotein level
$94.59
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
82550
Creatine kinase (cardiac enzyme) level, total
$75.22
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
82565
Blood creatinine level
$47.29
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
82725
Fatty acids measurement
$40.59
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
82947
Blood glucose (sugar) level
$59.96
Physician A LRH Medicare Beneficiary 2 9/13/2016 10/26/2016 83519
Measurement of substance using immunoassay
technique, by radioimmunoassay
$430.12
Physician A LRH Medicare Beneficiary 2 9/13/2016 10/26/2016 83520
Measurement of substance using immunoassay
technique
$92.73
Physician A LRH Medicare Beneficiary 2 9/13/2016 10/26/2016 83520
Measurement of substance using immunoassay
technique
$92.73
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
83525
Insulin measurement, total
$46.92
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
83695
Lipoprotein (A) level
$39.47
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 138 of 154 PageID #: 1021

139
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician A LRH Medicare Beneficiary 2 9/13/2016 10/26/2016 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$103.15
Physician A LRH Medicare Beneficiary 2 9/13/2016 10/26/2016 83704
Lipoprotein level, quantitation of lipoprotein
particle number(s)
$203.33
Physician A LRH Medicare Beneficiary 2 9/13/2016 10/26/2016 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$103.15
Physician A LRH Medicare Beneficiary 2 9/13/2016 10/26/2016 83880
Natriuretic peptide (heart and blood vessel
protein) level
$103.15
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
83921
Organic acid level
$121.40
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
84075
Phosphatase (enzyme) level, alkaline
$76.71
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
84443
Blood test, thyroid stimulating hormone (TSH)
$64.43
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
84450
Liver enzyme (SGOT), level
$15.64
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
84460
Liver enzyme (SGPT), level
$51.39
Physician A LRH Medicare Beneficiary 2 9/13/2016 10/26/2016 84520
Urea nitrogen level to assess kidney function,
quantitative
$40.22
Physician A
LRH
Medicare
Beneficiary 2
9/13/2016
10/26/2016
84550
Uric acid level, blood
$54.00
Physician A LRH Medicare Beneficiary 2 9/13/2016 10/26/2016 86141
Measurement C-reactive protein for detection of
infection or inflammation, high sensitivity
$102.41
Physician A LRH Medicare Beneficiary 3 5/9/2017 6/12/2017 G0483
Drug test(s), definitive; 22 or more drug class(es),
including metabolite(s) if performed
$607.52
Physician A
LRH
Medicare
Beneficiary 3
5/9/2017
6/12/2017
82610
Cystatin C (enzyme inhibitor) level
$26.11
Physician A
LRH
Medicare
Beneficiary 3
5/9/2017
6/12/2017
83525
Insulin measurement, total
$29.64
Physician A LRH Medicare Beneficiary 3 5/9/2017 6/12/2017 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$65.15
Physician A LRH Medicare Beneficiary 3 5/9/2017 6/12/2017 83880
Natriuretic peptide (heart and blood vessel
protein) level
$65.15
Physician A
LRH
Medicare
Beneficiary 3
5/9/2017
6/12/2017
84311
Chemical analysis using spectrophotometry (light)
$10.23
Physician A
LRH
Medicare
Beneficiary 3
5/9/2017
6/12/2017
84311
Chemical analysis using spectrophotometry (light)
$10.23
Physician A
LRH
Medicare
Beneficiary 3
5/9/2017
6/12/2017
84481
Thyroid hormone, T3 measurement, free
$38.57
Physician A
LRH
Medicare
Beneficiary 3
5/9/2017
6/12/2017
84550
Uric acid level, blood
$34.10
Physician A LRH Medicare Beneficiary 3 5/9/2017 6/12/2017 86376
Microsomal antibodies (autoantibody)
measurement
$39.04
Physician B LRH Medicare Beneficiary 4 1/18/2016 3/2/2016 81240
Gene analysis (prothrombin, coagulation factor II)
A variant
$125.00
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 139 of 154 PageID #: 1022

140
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician B LRH Medicare Beneficiary 4 1/18/2016 3/2/2016 81241
Gene analysis (coagulation factor V) Leiden
variant
$155.23
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
81401
Molecular pathology procedure level 2
$306.56
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
82306
Vitamin D-3 level
$125.13
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
82465
Cholesterol level
$41.71
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
82550
Creatine kinase (cardiac enzyme) level, total
$75.22
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
82565
Blood creatinine level
$47.29
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
82725
Fatty acids measurement
$40.59
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
82947
Blood glucose (sugar) level
$59.96
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
82985
Glycated protein level
$53.25
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
83036
Hemoglobin A1C level
$46.92
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
83090
Homocysteine (amino acid) level
$109.11
Physician B LRH Medicare Beneficiary 4 1/18/2016 3/2/2016 83519
Measurement of substance using immunoassay
technique, by radioimmunoassay
$430.12
Physician B LRH Medicare Beneficiary 4 1/18/2016 3/2/2016 83520
Measurement of substance using immunoassay
technique
$92.73
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
83525
Insulin measurement, total
$46.92
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
83695
Lipoprotein (A) level
$39.47
Physician B LRH Medicare Beneficiary 4 1/18/2016 3/2/2016 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$103.15
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
83701
Lipoprotein measurement
$75.60
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
83718
HDL cholesterol level
$24.95
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
83721
LDL cholesterol level
$29.05
Physician B LRH Medicare Beneficiary 4 1/18/2016 3/2/2016 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$103.15
Physician B LRH Medicare Beneficiary 4 1/18/2016 3/2/2016 83880
Natriuretic peptide (heart and blood vessel
protein) level
$103.15
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
83921
Organic acid level
$121.40
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
84075
Phosphatase (enzyme) level, alkaline
$76.71
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
84443
Blood test, thyroid stimulating hormone (TSH)
$64.43
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
84450
Liver enzyme (SGOT), level
$15.64
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
84460
Liver enzyme (SGPT), level
$51.39
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
84478
Triglycerides level
$46.18
Physician B LRH Medicare Beneficiary 4 1/18/2016 3/2/2016 84520
Urea nitrogen level to assess kidney function,
quantitative
$40.22
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 140 of 154 PageID #: 1023

141
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
84550
Uric acid level, blood
$54.00
Physician B
LRH
Medicare
Beneficiary 4
1/18/2016
3/2/2016
85384
Fibrinogen (factor 1) activity measurement
$41.71
Physician B LRH Medicare Beneficiary 4 1/18/2016 3/2/2016 86141
Measurement C-reactive protein for detection of
infection or inflammation, high sensitivity
$102.41
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
82040
Albumin (protein) level
$41.62
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
82465
Cholesterol level
$34.02
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
82550
Creatine kinase (cardiac enzyme) level, total
$61.36
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
82565
Blood creatinine level
$38.58
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
82947
Blood glucose (sugar) level
$48.91
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
83036
Hemoglobin A1C level
$38.28
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
83090
Homocysteine (amino acid) level
$89.01
Physician C LRH TRICARE Beneficiary 5 1/28/2016 5/20/2016 83520
Measurement of substance using immunoassay
technique
$75.64
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
83525
Insulin measurement, total
$38.28
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
83695
Lipoprotein (A) level
$32.21
Physician C LRH TRICARE Beneficiary 5 1/28/2016 5/20/2016 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$84.15
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
83701
Lipoprotein measurement
$61.66
Physician C LRH TRICARE Beneficiary 5 1/28/2016 5/20/2016 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$84.15
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
84075
Phosphatase (enzyme) level, alkaline
$62.58
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
84443
Blood test, thyroid stimulating hormone (TSH)
$52.55
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
84450
Liver enzyme (SGOT), level
$12.76
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
84460
Liver enzyme (SGPT), level
$41.92
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
84478
Triglycerides level
$37.67
Physician C LRH TRICARE Beneficiary 5 1/28/2016 5/20/2016 84520
Urea nitrogen level to assess kidney function,
quantitative
$32.80
Physician C
LRH
TRICARE
Beneficiary 5
1/28/2016
5/20/2016
84550
Uric acid level, blood
$44.05
Physician C LRH TRICARE Beneficiary 5 1/28/2016 5/20/2016 86141
Measurement C-reactive protein for detection of
infection or inflammation, high sensitivity
$83.54
Physician C LRH Medicare Beneficiary 6 2/11/2016 3/15/2016 81240
Gene analysis (prothrombin, coagulation factor II)
A variant
$125.00
Physician C LRH Medicare Beneficiary 6 2/11/2016 3/15/2016 81241
Gene analysis (coagulation factor V) Leiden
variant
$155.23
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
81401
Molecular pathology procedure level 2
$306.56
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 141 of 154 PageID #: 1024

142
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
82040
Albumin (protein) level
$51.02
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
82306
Vitamin D-3 level
$125.13
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
82465
Cholesterol level
$41.71
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
82550
Creatine kinase (cardiac enzyme) level, total
$75.22
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
82565
Blood creatinine level
$47.29
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
82725
Fatty acids measurement
$40.59
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
82947
Blood glucose (sugar) level
$59.96
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
83036
Hemoglobin A1C level
$46.92
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
83090
Homocysteine (amino acid) level
$109.11
Physician C LRH Medicare Beneficiary 6 2/11/2016 3/15/2016 83519
Measurement of substance using immunoassay
technique, by radioimmunoassay
$86.02
Physician C LRH Medicare Beneficiary 6 2/11/2016 3/15/2016 83520
Measurement of substance using immunoassay
technique
$92.73
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
83525
Insulin measurement, total
$46.92
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
83695
Lipoprotein (A) level
$39.47
Physician C LRH Medicare Beneficiary 6 2/11/2016 3/15/2016 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$103.15
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
83701
Lipoprotein measurement
$75.60
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
83718
HDL cholesterol level
$24.95
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
83721
LDL cholesterol level
$29.05
Physician C LRH Medicare Beneficiary 6 2/11/2016 3/15/2016 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$103.15
Physician C LRH Medicare Beneficiary 6 2/11/2016 3/15/2016 83880
Natriuretic peptide (heart and blood vessel
protein) level
$103.15
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
83921
Organic acid level
$121.40
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
84075
Phosphatase (enzyme) level, alkaline
$76.71
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
84443
Blood test, thyroid stimulating hormone (TSH)
$64.43
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
84450
Liver enzyme (SGOT), level
$15.64
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
84460
Liver enzyme (SGPT), level
$51.39
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
84478
Triglycerides level
$46.18
Physician C LRH Medicare Beneficiary 6 2/11/2016 3/15/2016 84520
Urea nitrogen level to assess kidney function,
quantitative
$40.22
Physician C
LRH
Medicare
Beneficiary 6
2/11/2016
3/15/2016
84550
Uric acid level, blood
$54.00
Physician C LRH Medicare Beneficiary 6 2/11/2016 3/15/2016 86141
Measurement C-reactive protein for detection of
infection or inflammation, high sensitivity
$102.41
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 142 of 154 PageID #: 1025

143
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
82040
Albumin (protein) level
$51.02
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
82465
Cholesterol level
$41.71
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
82550
Creatine kinase (cardiac enzyme) level, total
$75.22
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
82565
Blood creatinine level
$47.29
Physician D LRH Medicare Beneficiary 7 6/9/2016 9/8/2016 82627
Dehydroepiandrosterone (DHEA-S) hormone
level
$67.78
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
82670
Measurement of total estradiol (hormone)
$94.22
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
82725
Fatty acids measurement
$40.59
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
82947
Blood glucose (sugar) level
$59.96
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
82985
Glycated protein level
$53.25
Physician D LRH Medicare Beneficiary 7 6/9/2016 9/8/2016 83001
Gonadotropin, follicle stimulating (reproductive
hormone) level
$58.09
Physician D LRH Medicare Beneficiary 7 6/9/2016 9/8/2016 83002
Gonadotropin, luteinizing (reproductive hormone)
level
$66.29
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
83036
Hemoglobin A1C level
$46.92
Physician D LRH Medicare Beneficiary 7 6/9/2016 9/8/2016 83519
Measurement of substance using immunoassay
technique, by radioimmunoassay
$86.02
Physician D LRH Medicare Beneficiary 7 6/9/2016 9/8/2016 83520
Measurement of substance using immunoassay
technique
$92.73
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
83525
Insulin measurement, total
$46.92
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
83695
Lipoprotein (A) level
$39.47
Physician D LRH Medicare Beneficiary 7 6/9/2016 9/8/2016 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$103.15
Physician D LRH Medicare Beneficiary 7 6/9/2016 9/8/2016 83704
Lipoprotein level, quantitation of lipoprotein
particle number(s)
$197.37
Physician D LRH Medicare Beneficiary 7 6/9/2016 9/8/2016 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$103.15
Physician D LRH Medicare Beneficiary 7 6/9/2016 9/8/2016 83880
Natriuretic peptide (heart and blood vessel
protein) level
$103.15
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
83921
Organic acid level
$121.40
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84075
Phosphatase (enzyme) level, alkaline
$76.71
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84144
Progesterone (reproductive hormone) level
$63.31
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84270
Sex hormone binding globulin (protein) level
$65.91
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84403
Testosterone (hormone) level, total
$101.67
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84439
Thyroxine (thyroid chemical), free
$74.11
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 143 of 154 PageID #: 1026

144
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84443
Blood test, thyroid stimulating hormone (TSH)
$64.43
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84450
Liver enzyme (SGOT), level
$15.64
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84460
Liver enzyme (SGPT), level
$51.39
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84478
Triglycerides level
$46.18
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84481
Thyroid hormone, T3 measurement, free
$61.07
Physician D LRH Medicare Beneficiary 7 6/9/2016 9/8/2016 84520
Urea nitrogen level to assess kidney function,
quantitative
$40.22
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
84550
Uric acid level, blood
$54.00
Physician D
LRH
Medicare
Beneficiary 7
6/9/2016
9/8/2016
85384
Fibrinogen (factor 1) activity measurement
$41.71
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
80061
Blood test, lipids (cholesterol and triglycerides)
$11.69
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
82172
Apolipoprotein level
$41.67
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
82306
Vitamin D-3 level
$39.80
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
82550
Creatine kinase (cardiac enzyme) level
$4.13
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
82610
Cystatin C (enzyme inhibitor) level
$15.27
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
82725
Fatty acids measurement
$17.89
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
82947
Blood glucose (sugar) level
$2.79
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
82985
Glycated protein level
$20.27
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
83036
Hemoglobin A1C level
$13.05
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
83090
Homocysteine (amino acid) level
$22.68
Physician E BHD Medicare Beneficiary 8 1/6/2017 1/17/2017 83519
Measurement of substance using immunoassay
technique
$90.80
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
83525
Insulin measurement
$15.37
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
83695
Lipoprotein (A) level
$17.40
Physician E BHD Medicare Beneficiary 8 1/6/2017 1/17/2017 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$45.63
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
83704
Lipoprotein level
$42.41
Physician E BHD Medicare Beneficiary 8 1/6/2017 1/17/2017 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$45.63
Physician E BHD Medicare Beneficiary 8 1/6/2017 1/17/2017 83880
Natriuretic peptide (heart and blood vessel
protein) level
$45.63
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
83921
Organic acid level
$44.24
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
84550
Uric acid level, blood
$2.92
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
84681
C-peptide (protein) level
$27.98
Physician E
BHD
Medicare
Beneficiary 8
1/6/2017
1/17/2017
85384
Fibrinogen (factor 1) activity measurement
$11.42
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 144 of 154 PageID #: 1027

145
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician E BHD Medicare Beneficiary 8 1/6/2017 1/17/2017 86141
Measurement C-reactive protein for detection of
infection or inflammation
$17.40
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
80061
Blood test, lipids (cholesterol and triglycerides)
$22.87
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
82172
Apolipoprotein level
$52.94
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
82306
Vitamin D-3 level
$50.56
Physician E BHD TRICARE Beneficiary 9 4/24/2017 5/5/2017 82542
Chemical analysis using chromatography
technique
$24.68
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
82550
Creatine kinase (cardiac enzyme) level, total
$11.12
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
82610
Cystatin C (enzyme inhibitor) level
$23.22
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
82725
Fatty acids measurement
$22.74
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
82947
Blood glucose (sugar) level
$6.71
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
82985
Glycated protein level
$25.75
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
83036
Hemoglobin A1C level
$16.58
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
83090
Homocysteine (amino acid) level
$28.81
Physician E BHD TRICARE Beneficiary 9 4/24/2017 5/5/2017 83519
Measurement of substance using immunoassay
technique, by radioimmunoassay
$92.32
Physician E BHD TRICARE Beneficiary 9 4/24/2017 5/5/2017 83520
Measurement of substance using immunoassay
technique
$44.22
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
83525
Insulin measurement, total
$19.52
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
83695
Lipoprotein (A) level
$22.11
Physician E BHD TRICARE Beneficiary 9 4/24/2017 5/5/2017 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$57.97
Physician E BHD TRICARE Beneficiary 9 4/24/2017 5/5/2017 83704
Lipoprotein level, quantitation of lipoprotein
particle number(s)
$35.00
Physician E BHD TRICARE Beneficiary 9 4/24/2017 5/5/2017 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$57.97
Physician E BHD TRICARE Beneficiary 9 4/24/2017 5/5/2017 83880
Natriuretic peptide (heart and blood vessel
protein) level
$57.97
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
83921
Organic acid level
$56.20
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
83921
Organic acid level
$56.20
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
84550
Uric acid level, blood
$7.72
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
84681
C-peptide (protein) level
$35.55
Physician E
BHD
TRICARE
Beneficiary 9
4/24/2017
5/5/2017
85384
Fibrinogen (factor 1) activity measurement
$14.51
Physician E BHD TRICARE Beneficiary 9 4/24/2017 5/5/2017 86141
Measurement C-reactive protein for detection of
infection or inflammation, high sensitivity
$22.11
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 145 of 154 PageID #: 1028

146
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
80061
Blood test, lipids (cholesterol and triglycerides)
$112.84
Physician F LRH Medicare Beneficiary 10 10/6/2015 12/22/2015 81240
Gene analysis (prothrombin, coagulation factor II)
A variant
$353.78
Physician F LRH Medicare Beneficiary 10 10/6/2015 12/22/2015 81241
Gene analysis (coagulation factor V) Leiden
variant
$242.80
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
82172
Apolipoprotein level
$94.59
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
82306
Vitamin D-3 level
$125.13
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
82550
Creatine kinase (cardiac enzyme) level, total
$75.22
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
82565
Blood creatinine level
$47.29
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
82947
Blood glucose (sugar) level
$59.96
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
83036
Hemoglobin A1C level
$46.92
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
83090
Homocysteine (amino acid) level
$109.11
Physician F LRH Medicare Beneficiary 10 10/6/2015 12/22/2015 83519
Measurement of substance using immunoassay
technique, by radioimmunoassay
$430.12
Physician F LRH Medicare Beneficiary 10 10/6/2015 12/22/2015 83520
Measurement of substance using immunoassay
technique
$92.73
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
83525
Insulin measurement, total
$46.92
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
83695
Lipoprotein (A) level
$39.47
Physician F LRH Medicare Beneficiary 10 10/6/2015 12/22/2015 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$103.15
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
83701
Lipoprotein measurement
$75.60
Physician F LRH Medicare Beneficiary 10 10/6/2015 12/22/2015 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$103.15
Physician F LRH Medicare Beneficiary 10 10/6/2015 12/22/2015 83880
Natriuretic peptide (heart and blood vessel
protein) level
$103.15
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
83921
Organic acid level
$121.40
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
83921
Organic acid level
$121.40
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
84075
Phosphatase (enzyme) level, alkaline
$76.71
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
84443
Blood test, thyroid stimulating hormone (TSH)
$64.43
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
84450
Liver enzyme (SGOT), level
$15.64
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
84460
Liver enzyme (SGPT), level
$51.39
Physician F LRH Medicare Beneficiary 10 10/6/2015 12/22/2015 84520
Urea nitrogen level to assess kidney function,
quantitative
$40.22
Physician F
LRH
Medicare
Beneficiary 10
10/6/2015
12/22/2015
84550
Uric acid level, blood
$54.00
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 146 of 154 PageID #: 1029

147
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician F LRH Medicare Beneficiary 10 10/6/2015 12/22/2015 86141
Measurement C-reactive protein for detection of
infection or inflammation, high sensitivity
$102.41
Physician G
THD
Medicare
Beneficiary 11
2/5/2016
2/11/2016
80061
Blood test, lipids (cholesterol and triglycerides)
$17.88
Physician G
THD
Medicare
Beneficiary 11
2/5/2016
2/11/2016
82172
Apolipoprotein level
$41.38
Physician G THD Medicare Beneficiary 11 2/5/2016 2/11/2016 82542
Chemical analysis using chromatography
technique
$96.43
Physician G
THD
Medicare
Beneficiary 11
2/5/2016
2/11/2016
82652
Dihydroxyvitamin D, 1, 25 level
$51.39
Physician G
THD
Medicare
Beneficiary 11
2/5/2016
2/11/2016
82664
Electrophoresis, laboratory testing technique
$34.91
Physician G
THD
Medicare
Beneficiary 11
2/5/2016
2/11/2016
82725
Fatty acids measurement
$17.77
Physician G
THD
Medicare
Beneficiary 11
2/5/2016
2/11/2016
82777
Galectin-3 level
$29.36
Physician G
THD
Medicare
Beneficiary 11
2/5/2016
2/11/2016
83525
Insulin measurement, total
$15.26
Physician G THD Medicare Beneficiary 11 2/5/2016 2/11/2016 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$45.32
Physician G THD Medicare Beneficiary 11 2/5/2016 2/11/2016 83704
Lipoprotein level, quantitation of lipoprotein
particle number(s)
$42.12
Physician G THD Medicare Beneficiary 11 2/5/2016 2/11/2016 83880
Natriuretic peptide (heart and blood vessel
protein) level
$45.32
Physician G
THD
Medicare
Beneficiary 11
2/5/2016
2/11/2016
84311
Chemical analysis using spectrophotometry (light)
$18.66
Physician H THD Medicare Beneficiary 12 1/3/2017 1/12/2017 80053
Blood test, comprehensive group of blood
chemicals
$14.20
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
80061
Blood test, lipids (cholesterol and triglycerides)
$6.53
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
81401
Molecular pathology procedure level 2
$137.20
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
82172
Apolipoprotein level
$41.67
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
82306
Vitamin D-3 level
$36.53
Physician H THD Medicare Beneficiary 12 1/3/2017 1/12/2017 82542
Chemical analysis using chromatography
technique
$48.55
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
82607
Cyanocobalamin (vitamin B-12) level
$20.27
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
82610
Cystatin C (enzyme inhibitor) level
$18.28
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
82664
Electrophoresis, laboratory testing technique
$35.15
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
82725
Fatty acids measurement
$17.89
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
82747
Folic acid level, RBC
$23.24
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
82777
Galectin-3 level
$29.57
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
83090
Homocysteine (amino acid) level
$22.68
Physician H THD Medicare Beneficiary 12 1/3/2017 1/12/2017 83520
Measurement of substance using immunoassay
technique
$34.81
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 147 of 154 PageID #: 1030

148
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
83525
Insulin measurement, total
$15.37
Physician H THD Medicare Beneficiary 12 1/3/2017 1/12/2017 83704
Lipoprotein level, quantitation of lipoprotein
particle number(s)
$42.41
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
83735
Magnesium level
$9.01
Physician H THD Medicare Beneficiary 12 1/3/2017 1/12/2017 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$45.63
Physician H THD Medicare Beneficiary 12 1/3/2017 1/12/2017 83880
Natriuretic peptide (heart and blood vessel
protein) level
$45.63
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
83921
Organic acid level
$22.12
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84100
Phosphate level
$2.35
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84206
Proinsulin (pancreatic hormone) level
$23.94
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84311
Chemical analysis using spectrophotometry (light)
$9.40
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84311
Chemical analysis using spectrophotometry (light)
$9.40
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84378
Carbohydrate analysis, single quantitative
$3.87
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84439
Thyroxine (thyroid chemical), free
$12.12
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84443
Blood test, thyroid stimulating hormone (TSH)
$22.59
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84481
Thyroid hormone, T3 measurement, free
$22.78
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84482
Thyroid hormone, T3 measurement, reverse
$10.34
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84550
Uric acid level, blood
$2.24
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
84681
C-peptide (protein) level
$27.98
Physician H THD Medicare Beneficiary 12 1/3/2017 1/12/2017 85025
Complete blood cell count (red cells, white blood
cell, platelets), automated test and automated
differential white blood cell count
$10.45
Physician H
THD
Medicare
Beneficiary 12
1/3/2017
1/12/2017
86341
Islet cell (pancreas) antibody measurement
$23.26
Physician H THD Medicare Beneficiary 12 1/3/2017 1/12/2017 86376
Microsomal antibodies (autoantibody)
measurement
$19.56
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
80061
Blood test, lipids (cholesterol and triglycerides)
$22.71
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
82040
Albumin (protein) level
$8.39
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
82172
Apolipoprotein level
$52.56
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
82306
Vitamin D-3 level
$50.21
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
82550
Creatine kinase (cardiac enzyme) level
$11.05
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
82565
Blood creatinine level
$8.69
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
82947
Blood glucose (sugar) level
$6.66
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
82985
Glycated protein level
$25.57
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
83036
Hemoglobin A1C level
$16.46
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 148 of 154 PageID #: 1031

149
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
83090
Homocysteine (amino acid) level
$28.62
Physician I BHD TRICARE Beneficiary 13 1/24/2017 2/4/2017 83519
Measurement of substance using immunoassay
technique
$114.55
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
83525
Insulin measurement
$19.39
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
83695
Lipoprotein (A) level
$21.96
Physician I BHD TRICARE Beneficiary 13 1/24/2017 2/4/2017 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$57.57
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
83704
Lipoprotein level
$43.45
Physician I BHD TRICARE Beneficiary 13 1/24/2017 2/4/2017 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$57.57
Physician I BHD TRICARE Beneficiary 13 1/24/2017 2/4/2017 83880
Natriuretic peptide (heart and blood vessel
protein) level
$57.57
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
83921
Organic acid level
$55.82
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
83921
Organic acid level
$55.82
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
84075
Phosphatase (enzyme) level, alkaline
$8.78
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
84443
Blood test, thyroid stimulating hormone (TSH)
$28.50
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
84450
Liver enzyme (SGOT), level
$8.78
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
84460
Liver enzyme (SGPT), level
$8.99
Physician I BHD TRICARE Beneficiary 13 1/24/2017 2/4/2017 84520
Urea nitrogen level to assess kidney function,
quantitative
$6.70
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
84550
Uric acid level, blood
$7.68
Physician I
BHD
TRICARE
Beneficiary 13
1/24/2017
2/4/2017
85384
Fibrinogen (factor 1) activity measurement
$14.41
Physician I BHD TRICARE Beneficiary 13 1/24/2017 2/4/2017 86141
Measurement C-reactive protein for detection of
infection or inflammation
$21.96
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 80053
Blood test, comprehensive group of blood
chemicals
$9.27
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
80061
Blood test, lipids (cholesterol and triglycerides)
$11.78
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82172
Apolipoprotein level
$41.34
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82492
Chemical analysis
$24.09
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82492
Chemical analysis
$24.09
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82533
Cortisol (hormone) measurement, total
$21.75
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 82544
Chemical analysis using chromatography
technique
$24.09
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 82544
Chemical analysis using chromatography
technique
$24.09
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 149 of 154 PageID #: 1032

150
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82610
Cystatin C (enzyme inhibitor) level
$18.13
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 82627
Dehydroepiandrosterone (DHEA-S) hormone
level
$29.65
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82664
Electrophoresis, laboratory testing technique
$34.87
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82670
Measurement of total estradiol (hormone)
$37.26
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82679
Estrone (hormone) level
$33.28
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82725
Fatty acids measurement
$17.76
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82747
Folic acid level, RBC
$23.06
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
82777
Galectin-3 level
$29.33
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 83520
Measurement of substance using immunoassay
technique
$34.54
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
83525
Insulin measurement, total
$15.24
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 83698
Lipoprotein-associated phospholipase A2
(enzyme) level
$45.27
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 83704
Lipoprotein level, quantitation of lipoprotein
particle number(s)
$42.07
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
83735
Magnesium level
$8.93
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$45.27
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 83880
Natriuretic peptide (heart and blood vessel
protein) level
$45.27
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
83921
Organic acid level
$21.94
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
83970
Parathormone (parathyroid hormone) level
$55.05
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84140
Pregnenolone (reproductive hormone) level
$27.57
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84154
PSA (prostate specific antigen) measurement, free
$24.53
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84206
Proinsulin (pancreatic hormone) level
$23.76
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84270
Sex hormone binding globulin (protein) level
$28.99
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84311
Chemical analysis using spectrophotometry (light)
$18.66
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84378
Carbohydrate analysis, single quantitative
$3.84
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84403
Testosterone (hormone) level, total
$34.43
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84439
Thyroxine (thyroid chemical), free
$12.02
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84443
Blood test, thyroid stimulating hormone (TSH)
$22.41
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84481
Thyroid hormone, T3 measurement, free
$22.59
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84482
Thyroid hormone, T3 measurement, reverse
$10.26
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84550
Uric acid level, blood
$4.06
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 150 of 154 PageID #: 1033
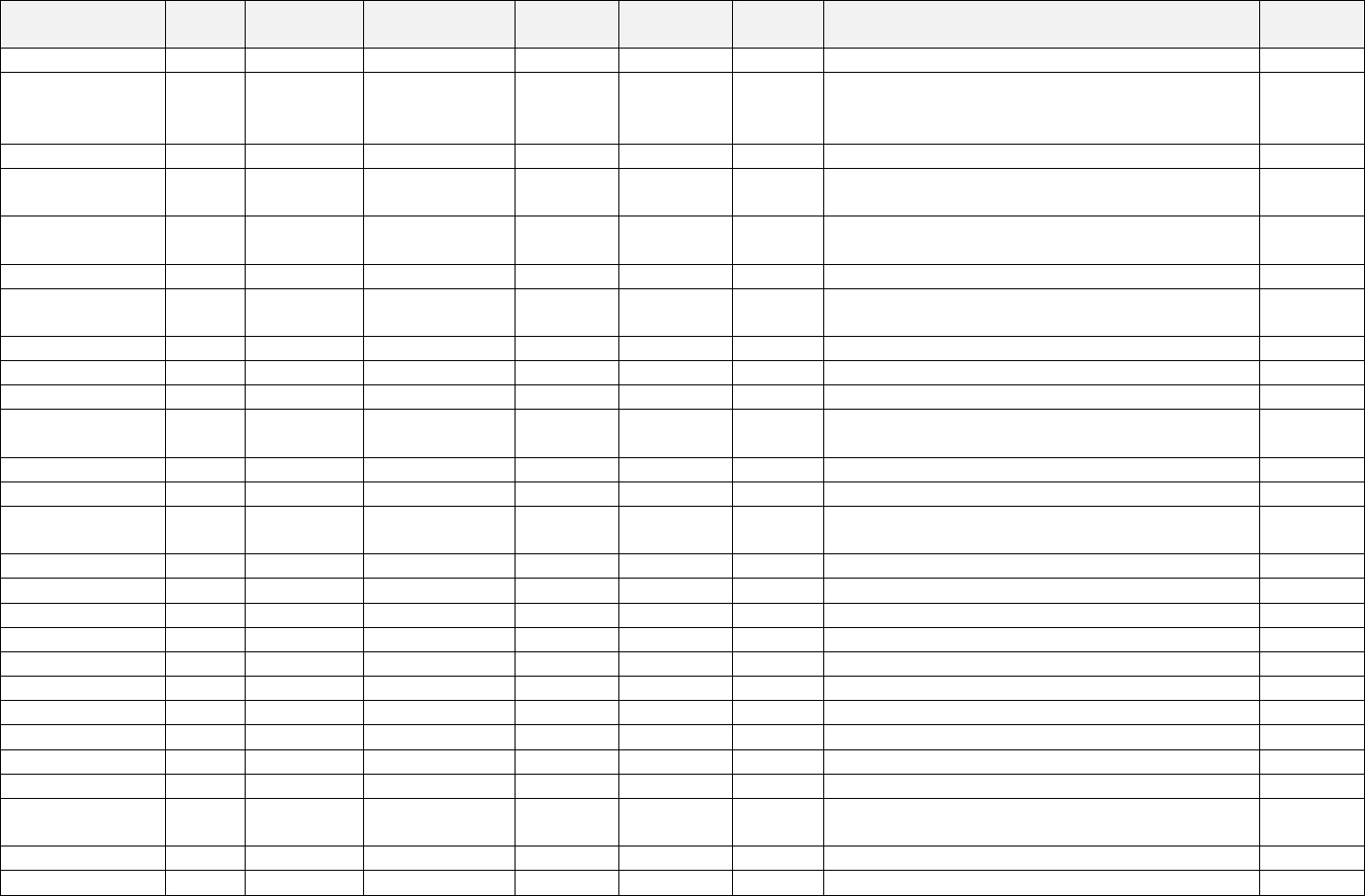
151
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
84681
C-peptide (protein) level
$27.75
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 85025
Complete blood cell count (red cells, white blood
cell, platelets), automated test and automated
differential white blood cell count
$10.37
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
86341
Islet cell (pancreas) antibody measurement
$23.08
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 86376
Microsomal antibodies (autoantibody)
measurement
$19.40
Physician J THD Medicare Beneficiary 14 10/2/2015 10/22/2015 86800
Thyroglobulin (thyroid protein) antibody
measurement
$21.22
Physician J
THD
Medicare
Beneficiary 14
10/2/2015
10/22/2015
G6047
Dihydrotestosterone
$34.43
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 80053
Blood test, comprehensive group of blood
chemicals
$9.28
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
80061
Blood test, lipids (cholesterol and triglycerides)
$11.81
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82172
Apolipoprotein level
$41.38
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82533
Cortisol (hormone) measurement, total
$21.77
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 82542
Chemical analysis using chromatography
technique
$120.54
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82607
Cyanocobalamin (vitamin B-12) level
$20.13
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82610
Cystatin C (enzyme inhibitor) level
$18.15
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 82627
Dehydroepiandrosterone (DHEA-S) hormone
level
$29.68
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82664
Electrophoresis, laboratory testing technique
$34.91
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82670
Measurement of total estradiol (hormone)
$37.30
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82679
Estrone (hormone) level
$33.32
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82725
Fatty acids measurement
$17.77
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82728
Ferritin (blood protein) level
$18.20
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82747
Folic acid level, RBC
$23.08
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82777
Galectin-3 level
$29.36
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
82985
Glycated protein level
$20.13
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
83036
Hemoglobin A1C level
$12.96
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
83090
Homocysteine (amino acid) level
$22.52
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 83520
Measurement of substance using immunoassay
technique
$34.55
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
83525
Insulin measurement, total
$15.26
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
83540
Iron level
$8.64
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 151 of 154 PageID #: 1034

152
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 83704
Lipoprotein level, quantitation of lipoprotein
particle number(s)
$42.12
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
83735
Magnesium level
$8.94
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
83789
Mass spectrometry (laboratory testing method)
$24.11
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 83876
Myeloperoxidase (white blood cell enzyme)
measurement
$45.32
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 83880
Natriuretic peptide (heart and blood vessel
protein) level
$45.32
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
83918
Organic acids level
$43.92
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
83970
Parathormone (parathyroid hormone) level
$55.11
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84140
Pregnenolone (reproductive hormone) level
$27.60
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84153
PSA (prostate specific antigen) measurement, total
$24.56
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84154
PSA (prostate specific antigen) measurement, free
$24.56
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84206
Proinsulin (pancreatic hormone) level
$23.77
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84270
Sex hormone binding globulin (protein) level
$29.02
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84311
Chemical analysis using spectrophotometry (light)
$18.66
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84378
Carbohydrate analysis, single quantitative
$3.84
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84403
Testosterone (hormone) level, total
$34.47
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84439
Thyroxine (thyroid chemical), free
$12.03
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84443
Blood test, thyroid stimulating hormone (TSH)
$22.43
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84481
Thyroid hormone, T3 measurement, free
$22.61
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84482
Thyroid hormone, T3 measurement, reverse
$10.27
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84550
Uric acid level, blood
$4.06
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
84681
C-peptide (protein) level
$27.78
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 85025
Complete blood cell count (red cells, white blood
cell, platelets), automated test and automated
differential white blood cell count
$10.38
Physician J
THD
Medicare
Beneficiary 14
1/14/2016
3/7/2016
86341
Islet cell (pancreas) antibody measurement
$23.10
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 86376
Microsomal antibodies (autoantibody)
measurement
$19.42
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 86800
Thyroglobulin (thyroid protein) antibody
measurement
$21.24
Physician J THD Medicare Beneficiary 14 1/14/2016 3/7/2016 G0480
Drug test(s), definitive, utilizing (1) drug
identification methods able to identify individual
drugs and distinguish between structural isomers
$78.34
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 152 of 154 PageID #: 1035

153
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
(but not necessarily stereoisomers), including, but
not limited to gc/ms (any type, single or tandem
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
80061
Blood test, lipids (cholesterol and triglycerides)
$12.33
Physician K THD Medicare Beneficiary 15 2/10/2015 4/28/2015 81240
Gene analysis (prothrombin, coagulation factor II)
A variant
$65.62
Physician K THD Medicare Beneficiary 15 2/10/2015 4/28/2015 81241
Gene analysis (coagulation factor V) Leiden
variant
$81.50
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
82040
Albumin (protein) level
$3.33
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
82533
Cortisol (hormone) measurement, total
$21.75
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
82607
Cyanocobalamin (vitamin B-12) level
$20.10
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
82610
Cystatin C (enzyme inhibitor) level
$18.13
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
82725
Fatty acids measurement
$17.76
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
82746
Folic acid level, serum
$19.61
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
82947
Blood glucose (sugar) level
$2.65
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
83090
Homocysteine (amino acid) level
$22.49
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
83525
Insulin measurement, total
$15.24
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
83695
Lipoprotein (A) level
$17.27
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
83701
Lipoprotein measurement
$33.10
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
83735
Magnesium level
$8.93
Physician K THD Medicare Beneficiary 15 2/10/2015 4/28/2015 83880
Natriuretic peptide (heart and blood vessel
protein) level
$45.27
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
84378
Carbohydrate analysis, single quantitative
$3.84
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
84403
Testosterone (hormone) level, total
$34.43
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
84550
Uric acid level, blood
$3.05
Physician K
THD
Medicare
Beneficiary 15
2/10/2015
4/28/2015
84681
C-peptide (protein) level
$27.75
Physician K THD Medicare Beneficiary 16 1/8/2016 1/15/2016 80053
Blood test, comprehensive group of blood
chemicals
$7.06
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
80061
Blood test, lipids (cholesterol and triglycerides)
$8.97
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
82172
Apolipoprotein level
$41.38
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
82248
Bilirubin level, direct
$3.38
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
82533
Cortisol (hormone) measurement, total
$21.77
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
82610
Cystatin C (enzyme inhibitor) level
$18.15
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
82652
Dihydroxyvitamin D, 1, 25 level
$51.39
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
82664
Electrophoresis, laboratory testing technique
$34.91
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
82725
Fatty acids measurement
$17.77
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 153 of 154 PageID #: 1036
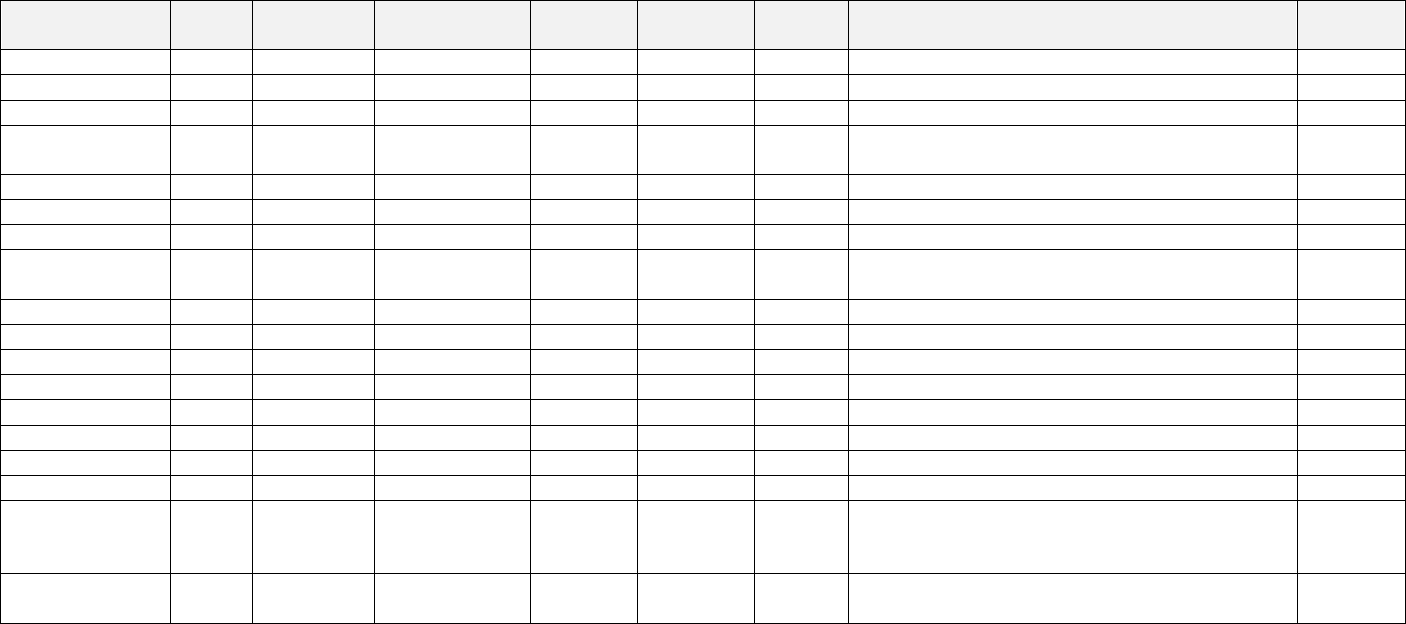
154
Referring HCP
Billing
Entity
Payor Beneficiary
2
Referral
Date
Claim
Date
3
CPT
Code
CPT Description Payment
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
82728
Ferritin (blood protein) level
$18.20
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
82747
Folic acid level, RBC
$23.08
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
83036
Hemoglobin A1C level
$12.96
Physician K THD Medicare Beneficiary 16 1/8/2016 1/15/2016 83520
Measurement of substance using immunoassay
technique
$17.28
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
83525
Insulin measurement, total
$15.26
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
83540
Iron level
$8.64
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
83735
Magnesium level
$8.94
Physician K THD Medicare Beneficiary 16 1/8/2016 1/15/2016 83880
Natriuretic peptide (heart and blood vessel
protein) level
$45.32
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
84100
Phosphate level
$3.23
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
84311
Chemical analysis using spectrophotometry (light)
$9.33
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
84378
Carbohydrate analysis, single quantitative
$3.84
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
84439
Thyroxine (thyroid chemical), free
$12.03
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
84443
Blood test, thyroid stimulating hormone (TSH)
$22.43
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
84481
Thyroid hormone, T3 measurement, free
$22.61
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
84550
Uric acid level, blood
$3.08
Physician K
THD
Medicare
Beneficiary 16
1/8/2016
1/15/2016
84681
C-peptide (protein) level
$27.78
Physician K THD Medicare Beneficiary 16 1/8/2016 1/15/2016 85025
Complete blood cell count (red cells, white blood
cell, platelets), automated test and automated
differential white blood cell count
$10.38
Physician K THD Medicare Beneficiary 16 1/8/2016 1/15/2016 86376
Microsomal antibodies (autoantibody)
measurement
$19.42
Case 4:16-cv-00547-ALM Document 57 Filed 01/31/22 Page 154 of 154 PageID #: 1037
