
1
PharmaSUG 2021 - Paper SI-083
Agile Project Management in Analysis and Reporting of Late Stage Clinical
Trials
Sarad Nepal, Uday Preetham Palukuru, Peikun Wu, Madhusudhan Ginnaram, Ruchitbhai Patel, Abhilash
Vasu Chimbirithy, Changhong Shi, Yilong Zhang, Merck & Co., Inc., Kenilworth, NJ, USA.
ABSTRACT
Analysis and reporting (A&R) in clinical trials requires careful planning especially when multiple projects
are ongoing at the same time in a clinical trial. The current practice typically uses a waterfall project
management structure that assumes one phase to be completed before another can start. When a clinical
trial nears data base lock, the time demands from statisticians and programmers increases due to both
planned and unplanned activities. Clinical study reports (CSR) that are required to be delivered in a short
period of time after database lock also contribute to the increase in challenges and time put towards the
project. Several agile management tools such as Confluence and Jira can be used to streamline the
project plan in an agile way. In this paper, we present details of how agile project management tools
were utilized to enhance team collaboration and ensure timely project delivery. We demonstrate this idea
using a recently completed clinical trial that involved three studies which were ongoing in
parallel and overlapping database lock and CSR delivery dates. A&R team comprising of several
programmers and statisticians collaborated and worked efficiently to deliver five CSRs and three
submission packages in short period of time. These deliverables were successfully submitted to multiple
regulatory agencies without any technical issues reported.
INTRODUCTION
Analysis and Reporting (A&R) is a key step in clinical research process. It requires careful planning to
generate all A&R deliverables and meet required timelines. It becomes challenging when multiple projects
are ongoing with overlapping timelines. All A&R programming done in clinical trials can be divided into four
system development lifecycle phases (SDLC). The Figure 1 provides an overview of these 4 phases.
Figure 1. The 4 phases of system development lifecycle in A&R programming in clinical trials.
There are number of project management techniques that are used routinely in late stage clinical trial
A&R. Of these project management techniques, the two most used techniques are Waterfall Project
Management (WPM) and Agile Project Management (APM). WPM is a more traditional approach that has
2
been successfully used in the industry for a long time. APM is relatively new development and is being
considered by the industry as a viable replacement to waterfall (Hass, 2007).
WPM assumes that there are predictable events affecting the project and the solutions to counter any
issues arising within the project lifecycle are already present. This project management technique allows
for a project to be split into multiple fixed phases, with each phase requiring the completion of the
previous phase (Bogdan-Alexandru et.al., 2019). In clinical trials, these phases can be broadly classified
as define, develop, validate and operation phase shown in the Figure 1.
The define phase is the most critical phase in the clinical trial development lifecycle as the specifications
for deliverables and resources needed to generate the deliverables are gathered and documented. Lead
programmer and statistician setup meeting with programming team to discuss the programming and
analysis requirements for the planned deliverables. Lead programmer in consultation with lead statistician
creates tasks related to deliverables in available tracking tool and assigns tasks (define, develop,
validate) to individual team members.
After the specifications are developed and individual deliverables task are assigned, the programming
development occurs in the develop phase. Lead programmer in consultation with lead statistician obtains
data as needed for program development as well as sets up the validation plan to ensure quality of
individual deliverables.
Programs developed in the develop phase are validated according to validation plan using requirement
specifications and validation plan in the validate phase. The programs are validated to achieve the
required quality and compliance of available standard procedures. The lead programmer in consultation
with lead statistician reviews completed deliverables and conducts test run of deliverables to ensure
quality. Operations phase is the final phase where the validated programs are executed with production
data to generate final version of required deliverables. Any changes or maintenance is done in this phase
as needed to address new requirements or issues. Lead programmer and statistician review the
deliverables generated by programming team in production area and setup meeting with requestors about
availability of deliverables and completion of programming activities. If changes are required, then
requestors communicate with lead programmer and specifications are updated as required.
The current practice typically uses a WPM structure that assumes one phase to be completed before
another can start. When a clinical trial nears data base lock, the time demands from statisticians and
programmers increases due to both planned and unplanned activities. Clinical study reports (CSR) that
are required to be delivered in a short period of time after database lock also contribute to the increase in
challenges and time put towards the project.
APM could be an alternative and efficient way to manage multiple ongoing clinical trials. APM is not one
specific method but an umbrella term for a collection of methods that share common characteristics. This
project management technique involves the active participation of both developers and project
stakeholders to identify the requirements as well as prioritize functionality of different phases of the
project. This technique is highly effective when the end goal of project is clear, and the customer actively
participate throughout the process (Hass, 2007 and Palmquist et.al., 2013).
Several agile management tools such as Confluence and Jira can be used to streamline the project plan
in an agile way. In this paper, we present details of how APM tools were utilized to enhance team
collaboration and ensure timely project delivery. We demonstrate this idea using a recently completed
clinical trial that involved three studies which were ongoing in parallel and overlapping database lock and
CSR delivery dates. A&R team comprising of several programmers and statisticians collaborated and
worked efficiently to deliver five CSRs and three submission packages in a short period of time. These
deliverables were successfully submitted to multiple regulatory agencies without any technical issues
reported.
MOTIVATION
Our company decided to design and implement a phase 3 clinical research trial. The results of the trial
were planned to be filed with multiple regulatory agencies regarding the outcome of the trials. Among this
large clinical trial, there were three clinical studies, which were ongoing in parallel and had overlapping

3
database lock and CSR delivery dates. Assimilation of all phase 3 studies data were required for pooled
data analyses in support of Common Technical Document (CTD) authoring for regulatory submissions.
The clinical trial was complex in scope due to the number of studies involved as well as the pooled data
analysis required in support of regulatory submissions. These studies all required individual clinical study
reports (CSR) at the end of each study completion as well as two Integrated Summary of Safety/Efficacy
(ISS/ISE) for pooled data analysis. These CSRs and ISS/ISE timelines overlapped significantly due to the
different start and end time of the individual studies. In total, there were 3 individual studies (P001, P002,
P003) and 2 pooled data analysis. The first pooled data analysis combined all three studies
(P001+P002+P003), the second pooled data analysis combined the last two studies (P002+P003). This
submission package contains 3 CSRs and 2 ISS/ISE. All these reports/summaries also required
associated electronic submission (esub) deliverables in preparation of regulatory submissions in
accordance with CTD guidelines.
The clinical trial followed the Clinical Data Interchange Standards Consortium (CDISC) guidelines for
clinical data including Study Data Tabulation Model (SDTM) v3.1.3 and Analysis Data Model (ADaM)
v1.0. For the A&R team this required generating ADaM datasets, Tables, Listings and Figures (TLFs) for
analysis purposes. The team also needed to generate define.xml, Analysis Data Reviewers Guide
(ADRG), Clinical Study Data Reviewer Guide (CSDRG), Analysis Result Metadata (ARM) and transport
(xpt) files for submission purposes. The total and individual numbers of deliverables of each
report/summary is summarized in Table 1. In order to generate all the deliverables described in Table 1,
the A&R team was assigned 5 statisticians and 10 programmers, including 1 lead statistician and 1 lead
programmer in-charge of the whole clinical trial.
Study/Pooled Data Analysis
ADaM Datasets
TLFs
P001 CSR
24
197
P002 CSR
17
200
P003 CSR
17
199
P002+P003 ISS/ISE
14
231
P001+P002+P003 ISS/ISE
14
222
Total
86
1049
Table 1. Number of deliverables associated with each individual study and pooled data analysis of
clinical trial.
The completion of A&R activities for the clinical program took approximately 18 months. There were
significant overlaps between the individual studies and pooled data analysis especially during the final
phases of report generation. The various phases in development of deliverables were planned in
Confluence and summarized in Figure 2.

4
Figure 2. Clinical trial A&R activities timeline by study/pooled data analysis.
AGILE MANAGEMENT IN CLINICAL TRIAL
The total number of deliverables required for analysis/submission purposes for the entire clinical trial was
daunting in nature due to the complexity of analysis. This was also true for the individual study or pooled
data analysis. The number of deliverables required was also compounded by the requirement to follow
study data standard guidelines for submissions. The guidelines specify several procedures to follow
ensuring conformance across submission packages. This increase in workload creates a greater
procedural burden than that for a non-submission package. The assignment of work priorities and
resource planning was also complicated due to the large A&R team assigned to the trial. The large team
size also increased the complexity in review of progress on both micro and macro levels.
To efficiently manage the complicated clinical trial, we adopted the agile approach for resource planning
and work assignment. The agile approach consists of iterative planning, development and delivery cycles
which allows a project team to constantly evaluate the deliverables and obtain immediate feedback from
stakeholders. After each successive cycle, the team learns and improves the deliverables as well as their
implementation methodologies. As with WPM, the agile approach requires a requirement definitions and
solutions design phase. Once the requirement at design phase is completed the development and testing
phases are implemented concurrently, and the feedback from the developers or testers are continuously
used to refine the requirements in the design phase. This allows the team to make immediate
modifications to the project requirements or resources allocation based on feedbacks from users as well
as customers. This iterative cycle ends with delivery of projects to customer once all feedback and
improvements are completed. The differences between the WPM and APM development lifecycles are
better illustrated in Figure 3.

5
Figure 3. The phases within development lifecycle of WPM and APM.
A&R activities in late stage clinical trials are both complex and demanding in nature due to the number of
different phases involved in the trial as well as the number of personnel from different functional areas. A
traditional WPM approach would only work for simpler A&R activities where the number of deliverables or
phases are few and well defined. For trials that encompass multiple deliverable packages or combination
of studies, the use of WPM would be cumbersome as the specification or requirements as well as
timelines would be in flux. The APM with its greater flexibility and collaboration provides a better solution
to the A&R activities. In APM, the project requirements continue to be refined based on the feedback from
stakeholders. It also incorporates flexibility with respect to timeline management. The use of APM also
helps in cost reduction compared to the WPM as making changes to a small part is cost effective.
In our clinical trial, we used Jira and Confluence to implement Agile management approach. Jira is a
project management tool that can be configured to fit any type of project. Team can start with the project
template provided by Jira or create their own custom workflow. Jira issues, also known as tasks, track
each piece of work that needs to pass through the workflow steps in order to be completed.
Administrators with customizable permissions determine who are assigned the issues. With all the
information in centralized location, reports can be generated to track progress and productivity of the
project and ensure timely completion of all tasks. Jira allows user to create a scrum board for APM where
the larger tasks are divided into short and small tasks to achieve productivity. This offer transparency
across project and status of every work item which enables teams to closely monitor the productivity over
time (Atlassian, 2021).
Confluence is a collaboration tool used to map out the project plan, milestones and clarify roles and
responsibilities within a project. In Confluence all the content lives in a dynamic document called a page.
Project plan, milestones, roles and responsibilities and other items of interest can be added in this page.
Teams can start with available project templates provided by Confluence or create their own using
available customization. It can be used with teams of any size and enables communication internally in
open and transparent way. The dynamic ability of Confluence pages gives a team a place to create,
capture and collaborate on project roadmap. Using Confluence, a team can make quick decisions, align
priorities and accomplish project milestones efficiently (Atlassian, 2021).
Jira in concert with Confluence enables teams to apply APM techniques efficiently. This is especially
useful in the context of late stage clinical trials where projects are often complex and have overlapping
timelines. The use of Jira and Confluence in late stage clinical trials helps in efficient allocation of
resources and improved communication about issues and potential solutions across team members in a
transparent manner. In order to efficiently manage all the A&R activities, a Jira project was created by the

6
lead statistician and lead programmer. This Jira project was setup after the preliminary define
phase described in the Introduction section was completed. The development and validation environment
for creating A&R deliverables was also setup prior to the Jira project creation.
The lead statistician and lead programmer were assigned as the project leads/administrators on the Jira
project. Once the project was setup the lead programmer assigned user access to the other personnel
assigned to the A&R activities. The users in the project could create/browse/search the issues within the
project. Only administrators could access the project administration section and manage user permissions
thereby limiting access to the project. The lead programmer in consultation with lead statistician also sets
up the data classification for the project to curtail any issues with data sensitivity. At the time of the
setup, all team members were asked to only discuss general trial information on Jira and refrain from
using any sensitive or confidential information within the Jira board. The changes made by the
administrator of the Jira project were automatically communicated to the users assigned to the projects
via email notification by Jira. A representative Jira board with weekly sprint and backlog sprint is shown in
Figure 4.
Figure 4. Jira board with weekly sprint and backlog sprint of the assigned tasks in the clinical trial.
Once the Jira project was setup, the lead statistician in consultation with lead programmer sets up epics.
Epics in Jira are a way to classify various sub-projects within a project. The epics corresponded to each
individual study or pooled analysis. The lead programmer in consultation with the programming and
statistician team members defines various tasks within each epic. The tasks within each epic are called
as issues within Jira. Based on feedback obtained during development and validation phases, additional
issues or modification of the existing issues can be done throughout the life of the project. Jira allows user
to import issues in batch. The lead programmer consolidates all the issues within a .csv file that is
then directly imported into Jira for assignment as issues within each epic. The .csv file contains inputs for
Issue Type, Summary, Epic, Priority, Due date and any other relevant fields. The imported issues are
automatically validated by Jira to ensure compatibility with Jira board. The Figure 4 also shows some of
the Epics and tasks that were setup in the clinical trial.
Once the issues are imported or created in Jira, the lead programmer in consultation with the lead
statistician then assigns tasks to individual team member. The lead programmer can also designate one
programmer as lead for each epic in order to efficiently track the progress of issues within each epic. The
issues within each epic can be assigned to a sprint within Jira. The sprint allows for grouping of issues

7
into a specific timeframe for the resolution of the issues. The concept of sprint can help the whole team
focus on specific tasks within a short period of time and identify gaps earlier. If an issue is not completed
in the current sprint, it can be assigned to a backlog. Backlogs in Jira help the trial leads to identify issues
that are lagging and assign priority to the completion of those issues. The priority of an issue from low to
medium to high can be assigned based on the requirements of the project. The sprints and backlogs in
the project were managed by the leads during a weekly project alignment meeting with the entire A&R
team. During the weekly project alignment meeting, each study member would report on the status of
open issues assigned to them and discuss bottlenecks and challenges. This weekly alignment meeting
ensured the smooth progress of the trial. This also enabled constant communication within the team on
the various lags or leads in the timeline of the trial as it progressed.
During the lifecycle of the clinical trial, the lead programmer and statistician participate in separate
weekly resource planning meeting to discuss the overall status of the trial. Jira reports about each epic,
issues assigned to users and resolution percentage of issues are generated by the lead programmer and
discussed with the lead statistician in these status meeting. This allows the trial leads to identify gaps in
task progression for the overall trial as well as communicate efficiently to the end customers about the
progression of the trial. The use of the Jira report on different sprint and backlogs also helps the trial
leads identify gaps in resource allocation of issues. It also allows trial leads to justify if additional
resources need to be assigned to the trial to ensure timely deliverables of the trial. The various tasks
assigned in sprints and epics of the clinical trial are illustrated in Jira report shown in Figure 5.
Figure 5. Jira report of various sprints and epics in the clinical trial.
VISUALIZATION OF CLINICAL TRIAL PROGRESS
The lead programmer and statistician are responsible for tracking the progress of the clinical trial
throughout the lifecycle of the trial. Using APM and Jira, various reports can be generated that help the
lead programmer and statistician visualize the progress of the clinical trial. These reports are highly
customizable and can be shared with the team in easy to understand formats. One of the reports
constantly used during the lifecycle of the clinical trial previously described was the distribution of total
tasks assigned to individual resources within the trial. Figure 6 shows a pie chart of all the tasks assigned
to the individual resources in Jira. This chart was communicated to internal and external team members
as a representation of the workload of the individual resource. This helped in reallocation of task within
the resources to achieve higher efficiency.

8
Figure 6. Jira report of total tasks assigned to individual resource assigned to a clinical trial.
Another Jira report that was used extensively in the clinical trial is shown in Figure 7. This created vs
resolved issue report was useful in tracking issue resolution over time. The report helped the lead
programmer and statistician make informed choices about the resources allocated to the trial and
potential gaps that need to be addressed. As can be seen from the Figure 7 there was a significant gap in
created vs. resolved at the time when the timelines for different deliverables were overlapping. But the
allocation of additional resources and changing priority of tasks assigned to individual resource helped
bridge the gap and ensured timely completion of the trial.

9
Figure 7. Jira report of total tasks created vs resolved in clinical trial over time.
Another commonly used Jira report to track the progress of the clinical trial was the cumulative flow
diagram as shown in Figure 8. This report helps the lead statistician and the lead programmer to visualize
the status of tasks assigned over time. This enables the tracking of trial completion and helps the lead
programmer and statistician in adjusting the priority of tasks in individual epic. As can be seen from the
figure there was a significant gap between tasks that were yet to be started (To Do) vs the tasks that were
completed (Done). This gap narrows and disappears towards the end of the trial when all tasks were
started and completed in the assigned timeline.
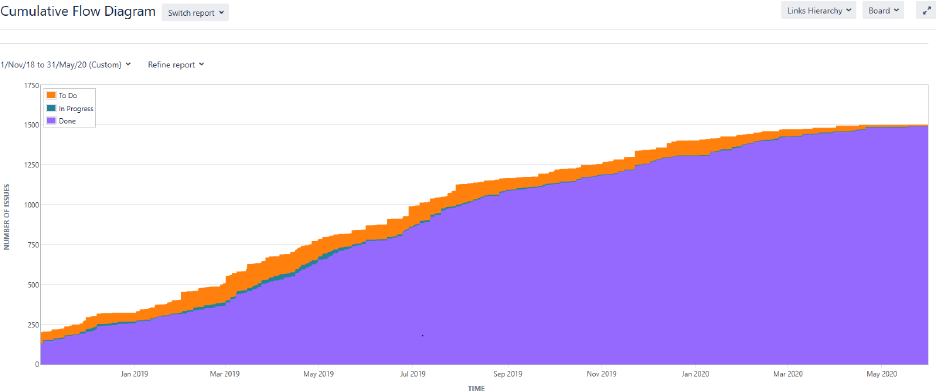
10
Figure 8. Jira report of issue status over time in clinical trial.
CONCLUSION
A&R activities involved in clinical trial development can be complex. This requires close collaboration of
statisticians and statistical programmers with clinical scientist, medical writer and data management, to
generate high quality CSRs and other deliverables. Although each organization might have a
methodology in place to plan and track the deliverables, most of the approaches involved traditional
waterfall management techniques. In this scenario, the lead statistician and lead programming might get
overwhelmed if there are multiple studies within a trial with overlapping timelines and multiple
programmers and statisticians associated with the trial. Additionally, with the advancement of outsourcing
deliverables to Contract Research Organizations (CRO) and location of resources in different
geographical areas, tracking individual progress on deliverables gets really challenging.
We illustrate how to use APM approach and tools in a real clinical trial to help alleviate burden on the lead
programmer and lead statistician and ensure transparency of the trial progress to every study team
member. The use of Jira and Confluence to track the progress of the trial both at a macro and a micro
level helped the lead programmer and lead statistician to efficiently manage the timely delivery in high-
quality. The workflow described in this paper also helped the individual team members increase their
contribution towards the trial completion by enabling transparent communication within the team as well
as tracking overall completion of assigned tasks. The use of epics and sprints within the clinical
trial helped in breaking down a complex project into a series of simple tasks with defined timelines for
each task. This helped the team members to better manage their priorities on the assigned task, as well
as helped the lead statistician and lead programmer by reducing micromanagement of individual team
member regarding a task. The use of epics and sprints also helped in refining of deliverables or
requirements when issues were found without impacting the timeline or deliverables of the entire trial.
The use of Confluence page helped the team members to accurately track the completion of various
milestones in the trial and manage resource allocation to deliverables that were lagging. In conclusion,
the use of APM technique in concert with Jira and Confluence helped in smooth progression of a complex
trial through the various phases of A&R activities. They also helped in reducing communication cost
and time demand on lead statistician and programmer for resource management and task allocation,
thereby increasing efficiency in managing the overall progress of the trial.
Although use of Jira and Confluence to implement APM in this clinical trial helped in the timely completion
of the trial, there are some avenues for improvement. In the future, we plan to implement a workflow to
automate Jira assignment using inhouse tools thereby further reducing time demands on lead statistician
and lead programmer for task assignments. We also plan to integrate cross functional teams including
clinical and medical writing teams into the A&R activities by allowing access to the Jira board and

11
Confluence pages, thereby allowing seamless communication within the entire trial team. Additionally, we
plan to expand the use of Jira and Confluence in internal standard macro package development.
REFERENCES
Atlassian. (2021, Jan 1). Confluence Basics. Retrieved Feb 18, 2021, from https://www.atlassian.com:
https://www.atlassian.com/software/confluence/guides/get-started/confluence-overview#hosting-options
Atlassian. (2021, Jan 1). What is Jira used for? Retrieved Feb 18, 2021, from https://www.atlassian.com/:
https://www.atlassian.com/software/jira/guides/use-cases/what-is-jira-used-for#Jira-for-requirements-&-
test-case-management
Bogdan-Alexandru, A., Andrei-Cosmin, C.-P., Sorin-Catalin, G., & Costin-Anton, B. (2019, May). A Study
on Using Waterfall and Agile Methods in Software Project Management. Journal of Information Systems
and Operations Management, 13(1), 125-135. Retrieved Feb 18, 2021
Hass, K. B. (2007, May). The Blending of Traditional and Agile Project Management. PM World Today,
IX(V), 1-8. Retrieved Feb 2021
Palmquist, S., Lapham, M. A., Miller, S., Chick, T., & Ozkaya, I. (2013, Oct). Parallel Worlds: Agile and
Waterfall. Technical Report CMUSEI-13-TN-21, 1-101.
ACKNOWLEDGMENTS
The authors would like to thank management teams from Merck & Co., Inc., Kenilworth, NJ, USA, for their
advice on this paper/presentation.
CONTACT INFORMATION
Your comments and questions are valued and encouraged. Contact the authors at:
Sarad Nepal
Merck & Co., Inc., Kenilworth, NJ, USA
E-mail: sarad.nepal@merck.com
Uday Preetham Palukuru, Ph.D.
Merck & Co., Inc., Kenilworth, NJ, USA
E-mail: preetham.paluku[email protected]
Yilong Zhang, Ph.D.
Merck & Co., Inc., Kenilworth, NJ, USA
E-mail: yilong.zhang@merck.com
Any brand and product names are trademarks of their respective companies.
