
Journal of Parenteral and Enteral
Nutrition
Volume 41 Number 1
January 2017 15 –103
© 2016 American Society
for Parenteral and Enteral Nutrition
DOI: 10.1177/0148607116673053
jpen.sagepub.com
Consensus Recommendation
ASPEN Safe Practices for Enteral Nutrition Therapy
Joseph I. Boullata, PharmD, RPh, BCNSP, FASPEN, FACN
1
;
Amy Long Carrera, MS, RD, CNSC, CWCMS
2
;
Lillian Harvey, MD, FACS, CNSC
3
; Arlene A. Escuro, MS, RD, LD, CNSC
4
;
Lauren Hudson, MS, RD, LDN
5
; Andrew Mays, PharmD
6
;
Carol McGinnis, DNP, RN, CNS, CNSC
7
;
Jacqueline J. Wessel, MEd, RDN, CNSC, CSP, CLE
8
;
Sarita Bajpai, PhD, RD, CD, CNSC
9
; Mara Lee Beebe, RD, LD, CNSC
10
;
Tamara J. Kinn, MS, RD, LDN, CNSC
11
;
Mark G. Klang, MS, RPh, BCNSP, PhD
12
; Linda Lord, NP, ACNP-BC, CNSC
13
;
Karen Martin, MA, RDN, LD, FAND
14
;
Cecelia Pompeii-Wolfe, RD, LDN, CNSC
15
; Jackie Sullivan, MS, RDN, CD
16
;
Abby Wood, RD, LD, CNSC
17
; Ainsley Malone, MS, RD, CNSC, FASPEN
18
; and
Peggi Guenter, PhD, RN, FAAN
18
; ASPEN Safe Practices for Enteral Nutrition
Therapy Task Force, American Society for Parenteral and Enteral Nutrition
Abstract
Enteral nutrition (EN) is a valuable clinical intervention for patients of all ages in a variety of care settings. Along with its many outcome
benefits come the potential for adverse effects. These safety issues are the result of clinical complications and of process-related errors.
The latter can occur at any step from patient assessment, prescribing, and order review, to product selection, labeling, and administration.
To maximize the benefits of EN while minimizing adverse events requires that a systematic approach of care be in place. This includes
open communication, standardization, and incorporation of best practices into the EN process. This document provides recommendations
based on the available evidence and expert consensus for safe practices, across each step of the process, for all those involved in caring
for patients receiving EN. (JPEN J Parenter Enteral Nutr. 2017;41:15-103)
Keywords
enteral nutrition; enteral access; enteral formulas; nutrition; safety
Table of Contents
Introduction 15
Assessment and Recommendations 18
Prescribing and Communicating the
Enteral Nutrition Order 22
Review of the Enteral Nutrition Order 31
Enteral Access 36
Procure, Select/Prepare, Label, and Dispense EN 48
Administration: General 59
Administration: EAD Patency 74
Medication Delivery via Enteral Access Devices 77
Complication Avoidance and Error Reporting 85
Monitoring and Reassessment 92
Transition of Care 95
Documentation and Quality Review Issues 100
Common Terms and Abbreviations Used
Throughout the Document
Blenderized tube feeding (BTF)
Computerized prescriber order entry (CPOE)
Electronic health record (EHR)
Enteral access device (EAD)
Enteral nutrition (EN)
Gastric residual volume (GRV)
Gastrointestinal (GI)
Head of bed (HOB)
Human breast milk (HBM)
Intensive care unit (ICU)
Parenteral nutrition (PN)
Introduction
Enteral nutrition (EN) refers to the system of providing nutri-
tion directly into the gastrointestinal (GI) tract bypassing the
oral cavity.
1
Each year in the United States, this nutrition sup-
port modality is used in 250,000 hospitalized patients annually
from infants to older adults.
2
EN is also widely used in sub-
acute, rehabilitation, long-term care, and home settings. For
the purposes of this document, EN will include those nutrient
formulas and human breast milk (HBM) delivered through an
enteral access device (EAD).
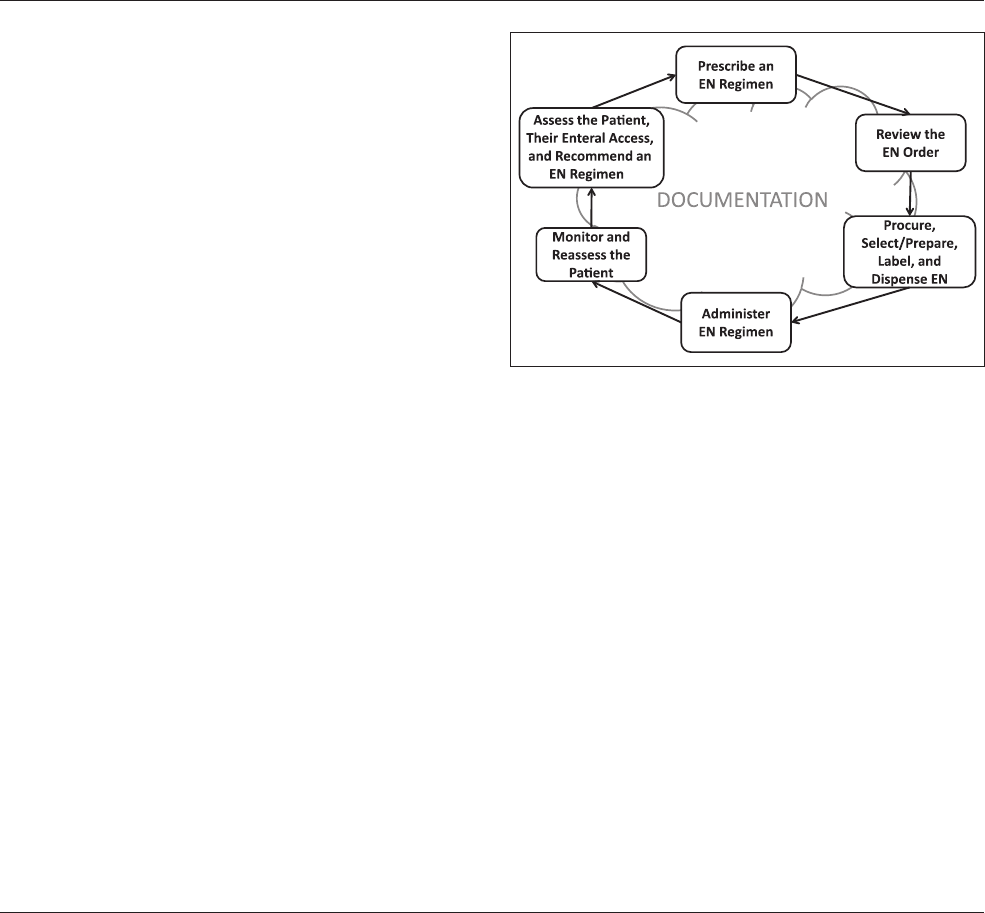
16 Journal of Parenteral and Enteral Nutrition 41(1)
The EN process (Figure 1) is the system within which EN is
used. This involves a number of major steps: the initial patient
assessment, the recommendations for an EN regimen, the
selection of the EAD, the EN prescription, the review of the
EN order, the product selection or preparation, the product
labeling and dispensing, the administration of the EN to the
patient, and the patient monitoring and reassessment, with doc-
umentation at each step as required. This process requires a
multidisciplinary team of competent clinicians working in con-
cert to provide safe nutrition care.
3
Although clinician competence is assumed in the EN Use
Process, an inherent risk of clinical complications is related to
EN and the formulas used, as well as potential errors at each
step in the process. Serious adverse events, including fatalities,
can occur when lapses allow for errors.
1,4
These types of
adverse events include the following:
Clinical complications of using EN such as GI compli-
cations, refeeding syndrome, or gut ischemia
Process-related errors, including those associated with
process steps, such as administration errors and
misconnections
Optimal communication and standardization across all
steps of the EN Use Process is a risk management strategy.
3
To
reduce the risk of adverse events and improve patient safety,
effective communication among all members of the multidis-
ciplinary team is necessary throughout the process.
4
Collectively, team members must also develop and adhere to
policies and standardized procedures for daily practice and
decision making related to patient care. Standardization does
not refer to, and should not lead to, a one-size-fits-all strategy
for patient care. Instead, it refers to the development and
implementation of technical and practice standards into a pro-
cess so that all healthcare providers deliver the same level of
safe care.
5
Opportunities exist for standardization across the
EN process (eg, EN order templates). Process standardization
may include independent double-checks and automation with
forcing functions to help improve EN safety. Policies include
the organization’s mechanisms to maintain competency of
individual clinicians involved in EN.
Methodology
This document focuses on safe practices for EN therapy. The
objective is to provide recommendations based on either evi-
dence (when available) or expert consensus that supports safe
practices by clinicians who recommend, prescribe, review, pre-
pare, administer, and/or monitor patients receiving EN therapy
and by their supporting organizational structures. Indications
for EN and the ethics surrounding the use of EN are outside of
the scope of this document.
To develop this document, an interdisciplinary group of
American Society for Parenteral and Enteral Nutrition
(ASPEN) experts identified key questions related to EN
Figure 1. The Enteral Nutrition (EN) Use Process.
From
1
Clinical Nutrition Support Services, Hospital of the University of Pennsylvania and Department of Nutrition, Drexel University, Philadelphia,
Pennsylvania, USA;
2
Shield Healthcare, Valencia, California, USA;
3
Northshore University Hospital, Manhasset, New York, and Hofstra University
NorthWell School of Medicine, Garden City, New York, USA;
4
Digestive Disease Institute Cleveland Clinic Cleveland, Ohio, USA;
5
Hospital of the
University of Pennsylvania, Philadelphia, Pennsylvania, USA;
6
Baptist Health Systems and University of Mississippi School of Pharmacy, Jackson,
Mississippi, USA;
7
Sanford University of South Dakota Medical Center, Sioux Falls, South Dakota, USA;
8
Cincinnati Children’s Hospital Medical
Center, Cincinnati, Ohio, USA;
9
Indiana University Health, Indianapolis, Indiana, USA;
10
Mount Carmel West Hospital, Columbus, Ohio, USA;
11
Loyola University Medical Center, Maywood, Illinois, USA;
12
Memorial Sloan Kettering Cancer Center, New York, New York, USA;
13
University
of Rochester Medical Center, Rochester, New York, USA;
14
University of Texas Center for Health Sciences at San Antonio, San Antonio, Texas, USA;
15
University of Chicago, Medicine Comer Children’s Hospital, Chicago, Illinois, USA;
16
Aurora Lakeland Medical Center, Elkhorn, Wisconsin, USA;
17
Baylor University Medical Center, Dallas, Texas, USA; and
18
American Society for Enteral and Parenteral Nutrition, Silver Spring, Maryland, USA.
Financial disclosure: None declared.
Conflicts of interest: L. Harvey and J. J. Wessel are members of the Abbott Nutrition Speakers Bureau. M. L. Beebe is a member of the Nutricia
Speakers Bureau. L. Lord is a member of Nestlé Nutrition and Bard. All other authors have no conflicts of interest to report.
Received for publication June 14, 2016; accepted for publication September 14, 2016.
This article originally appeared online on November 10, 2016.
Corresponding Author:
Peggi Guenter, PhD, RN, FAAN, ASPEN, 8630 Fenton St, Suite 412, Silver Spring, MD 20910, USA.
Email: [email protected]

Boullata et al 17
practice issues with safety implications. These questions were
then grouped into relevant sections, including patient assess-
ment, EN prescribing, order review, EN access, product han-
dling, administration, monitoring and reassessment, and
transition of care. The term order is used throughout the docu-
ment to refer to an EN prescription or the act of prescribing
EN. Administration was further divided to focus on tube
patency, medications, and complications, as well as general
approaches. A number of topics crossed sections. These are
addressed in depth in only one section and cross-referenced
elsewhere. Redundancy was built in purposefully as users will
likely go to a specific section for guidance.
The experts contributed to the sections with which they
had the most familiarity and experience. Under the direction
of a section leader, the authors performed an English-language
literature search using multiple terms relevant to the section
and questions posed. The experts then reviewed the available
literature and weighed risks against benefits to come to a set
of best practice recommendations for each question. Each set
of practice recommendations is followed by the rationale,
which cites relevant references. The sections that comprise
this document were reviewed in their entirety by task force
members. Discussions and consensus took place to arrive at
the final recommendations. This document has undergone
internal and external review, including approval by the ASPEN
Board of Directors.
The recommendations within this document are intended
for discussion and adoption over time by organizations
involved in the delivery of EN. These recommendations are
not intended to supersede the judgment of the healthcare pro-
fessional or employing institution based on the circumstances
of the individual patient.
References
1. Bankhead R, Boullata J, Brantley S, et al. Enteral nutrition practice recom-
mendations. JPEN J Parenter Enteral Nutr. 2009;33(2):122-167.
2. Agency for Healthcare Research and Quality. All listed ICD-9CM pro-
cedure code for enteral nutrition infusion 2013. Healthcare Utilization
Project (HCUP) National Inpatient Survey. www.hcupnet.ahrq.gov.
Accessed January 2016.
3. Hudson LM, Boullata JI. A quality improvement case report: an institu-
tion’s experience in pursuing excellence in parenteral nutrition safety.
JPEN J Parenter Enteral Nutr. 2014;38(3):378-384.
4. Malone AM, Seres DS, Lord L. Challenges and complications with enteral
nutrition. In: Mueller CM, ed. The Science and Practice of Nutrition
Support: A Case Based Curriculum. 3rd ed. Silver Spring, MD: American
Society for Enteral and Parenteral Nutrition; 2012:218-233.
5. Boullata JI. Safe practices for enteral and parenteral nutrition. In: Seres
DS, Van Way CW, eds. Nutrition Support for the Critically Ill. Cham,
Switzerland: Springer International Publishing; 2016:229-241.
Appendix 1. Water
Due to the repeated use of water throughout the enteral use
process, this appendix will delineate the terms and defini-
tions for the appropriate use of water terms. Reports in the
lay press about water contamination are giving clinicians
and patients a reason to pay closer attention to the source of
their water. For the patient receiving EN, there are multiple
points of interface with water and therefore will be discussed
here briefly. Water is used to hydrate the patient, flush the
EAD, and dilute medication and powdered formula.
Clinicians should be familiar with the terms used when
describing water (Table A1).
1
Regulations for drinking water
(Environmental Protection Agency) and bottled water (Food
and Drug Administration) are limited in the number of con-
taminants regulated and threshold concentrations allowed.
2,3
So although most drinking water may be considered safe for
healthy individuals, the types and concentrations of contami-
nants may pose risks to patients requiring EN. Contaminants
may be chemical or biologic; pathogenic microorganisms are
included in the latter. Water contaminated with pathogens
has been associated with colonization and infection with out-
breaks attributed to the water supply.
4–10
A source of sterile
water (eg, sterile water for irrigation) is considered best
practice for the immunocompromised patient and for recon-
stituting powdered enteral formula. The same water could be
used for preparing (diluting, reconstituting, compounding)
medication because it is an example of purified water (ie,
contaminant free), even though the sterility is not required.
The same water (ie, sterile water for irrigation) could even
be used for flushing the EAD and hydrating the patient when
the degree of chemical contamination of the drinking water
is unknown or excessive.
References
1. Boullata JI. Enteral nutrition practice: the water issue. Support Line.
2010;32(3):10-17.
2. United States Environmental Protection Agency.
3. United States Food and Drug Administration.
4. Venezia RA, Agresta MD, Hanley EM, et al. Nosocomial legio-
nellosis associated with aspiration of nasogastric feedings
diluted in tap water. Infect Control Hosp Epidemiol. 1994;15:
529-533.
5. Bert F, Maubec E, Bruneau B, et al. Multi-resistant Pseudomonas
aeruginosa outbreak associated with contaminated tap water
in a neurosurgery intensive care unit. J Hosp Infect. 1998;39:
53-62.
6. Anaissie EJ, Penzak SR, Dignani MC. The hospital water supply as a
source of nosocomial infections: a plea for action. Arch Intern Med.
2002;162: 1483-1492.
7. Vonberg RP, Eckmanns T, Bruderek J, et al. Use of terminal tap water
filter systems for prevention of nosocomial legionellosis. J Hosp
Infect. 2005;60: 159-162.
8. Johansson RJH, Andersson K, Wiebe T, et al. Nosocomial transmis-
sion of Legionella pneumophila to a child from a hospital’s cold-
water supply. Scan J Infect Dis. 2006;38:1023-1027.
9. Rogues AM, Boulestreau H, Lasheras A, et al. Contribution of tap
water to patient colonization with Pseudomonas aeruginosa
in a medical intensive care unit. J Hosp Infect. 2007;67:
72-78.
10. Garvey MI, Bradley CW, Tracey J, Oppenheim B. Continued trans-
mission of Pseudomonas aeruginosa from a wash hand basin tap in a
critical care unit. J Hosp Infect. 2016;94:8-12.

18 Journal of Parenteral and Enteral Nutrition 41(1)
Section 1. Assessment and
Recommendations
Background
EN is a complex therapy that may be associated with adverse
events. Therefore, before making any recommendations about
its use, a qualified nutrition clinician must evaluate indications
and weigh risks and benefits for each patient who may be a
candidate for this therapy. Nutrition assessment is a compre-
hensive approach to collecting and analyzing data from the
patient (history, physical exam, anthropometrics, laboratory,
and other tests) to diagnose any nutrition-related problem for
which nutrition intervention may be appropriate. In both the
adult and pediatric population, diagnosing malnutrition is
essential to promote improved outcomes.
1,2
A documented care
plan with consistent recommendations will follow the assess-
ment. The first goal is to evaluate the indication for EN.
Additional objectives of the assessment are to estimate macro-
nutrient, fluid, and micronutrient needs; determine the most
appropriate formula and route of administration; identify barri-
ers to tolerance; and prevent or ameliorate potential adverse
events, including GI intolerance, and metabolic and/or fluid
disturbances. Meeting these objectives requires a thorough
understanding of the patient’s overall condition. By making the
process of organizing and evaluating data as efficient as pos-
sible, institutions allow all members of the patient care team to
access the relevant information about EN recommendations;
thus, the electronic health record (EHR) may facilitate essen-
tial documentation and communication processes.
Question 1.1. What factors need to be included in the
overall nutrition assessment to determine the safety
and appropriateness of EN?
Practice Recommendations
1. Collect and organize relevant data on patient history,
physical exam, anthropometrics, laboratory values,
and other tests.
a. Patient history includes clinical diagnoses, past and
current medical and surgical interventions,
medications, dietary supplements, nutrition history,
social history, religious background, potential
ethical dilemmas, and mental status challenges.
b. Physical exam includes GI function assessment
and existing access devices as well as nutrition-
focused physical findings.
c. Anthropometrics includes height, weight, body
mass index (BMI), growth chart z scores, and any
available objective measures of body composition
or changes in any of these parameters.
d. Laboratory values and other test findings include
all relevant blood (eg, comprehensive metabolic
panel) and urinary tests regardless of whether the
findings are normal or abnormal, functional tests,
radiologic findings, or predictive scores such as
the Nutritional Risk Index.
2. Evaluate patient data to determine nutrition status, any
nutrition-related problem (real or potential), indication
for nutrition interventions via the enteral route, and
estimated energy, protein, fluid, and micronutrient
needs based on the patient’s status or accepted standards.
Rationale
Each of the recommended types of nutrition assessment data
provides essential information about whether EN is indicated
and can be administered safely. Nutrition status, including
presence or risk of malnutrition, also influences the effective-
ness and safety of implementing EN administration.
Patient history. The success of EN therapy depends on the
patient’s clinical state and disease process. A review of clinical
diagnoses and surgical/medical history will capture information
that has bearing on the patient’s ability to tolerate EN (Table 1).
3–8
A thorough social and nutrition history can determine if the
patient is at risk for refeeding syndrome due to recent anorexia or
food insecurity. This part of the assessment can also identify
nutrient intolerance or allergy, which could result in an adverse
Table A1. Water and Enteral Nutrition (EN) Use.
Term Definition Use in Patient Receiving EN
Source water Nonsaline, freshwater found on the surface (eg, lakes) or in the ground
(eg, aquifers)
No
Distribution water Water flowing from site of storage (eg, municipal treatment facility,
storage tank, or well) to point of use (ie, “tap” water)
Yes, for water flushes depending
on the degree of contaminants
Drinking water Distribution water and bottled water Yes, for water flushes depending
on the degree of contaminants
Purified water Contaminant free after treatment steps (eg, distillation, ultrafiltration,
UV light)
Yes, for medication preparation
Sterile water Purified water free of microorganisms and pyrogens Yes, for reconstituting powdered
formula
UV, ultraviolet.

Boullata et al 19
reaction to an EN product. The clinician evaluates GI symptoms
that may affect EN tolerance, such as nausea, bloating, diarrhea,
excessive ostomy output, constipation, abdominal discomfort or
pain, and reflux. Constipation is associated with early satiety and
feeding intolerance in addition to difficulty weaning from the
ventilator, related to an increase in intra-abdominal pressure.
9
Fecal impaction, obstruction, and ileus identified radiologically
will also affect EN tolerance. The nutrition clinician should also
note the presence of existing access devices or plans for EAD
placement and the appropriateness of these plans.
Prescribed medications that may affect safety and tolerance
of EN should be considered. For example, liquid medications
containing sorbitol may cause loose stools and abdominal dis-
comfort, leading to cessation of the feeding. Enteral feeding
administration should be rate adjusted and held with provision
of medications known to interact with formula or clog the
EAD. Medications should be scheduled for administration in
conjunction with the feeding regimen. At all times, a flushing
protocol should be in place to prevent formula-drug interaction
and device clogging. Hemodynamic instability and the need
for vasopressors increase the risk of gut ischemia, and the use
of EN should be considered cautiously in these patients.
6
Laboratory values and other test data. Closer review of perti-
nent laboratory values is an important component of the nutri-
tion assessment. Attention to hydration status, using available
markers such as urea nitrogen and urine sodium as well as fluid
intake and output, helps identify appropriate formula selection
and free water needs. Visceral proteins, including prealbumin,
in the presence of inflammatory biomarkers (eg, C-reactive
protein) may be useful as markers of inflammation and disease
severity as well as predictors of morbidity and mortality for
some populations.
10,11
However, these protein levels are not
indicative of nutrition status.
12
Anthropometry. Anthropometry, including weight and weight
history, is assessed to identify an adequate and appropriate feed-
ing regimen and to determine the presence or risk of malnutri-
tion. Unintentional weight loss is well established as an indicator
of malnutrition.
13
Malnutrition is associated with increased risk
of pneumonia, Clostridium difficile infection, pressure ulcers,
and postoperative complications.
14
In pediatrics, anthropometry
includes weight for age, length for age, and head circumference
for age and weight for length until 36 months. From age 2–20
years, weight for age, standing height for age, and BMI are
assessed. Plotting children on the appropriate growth chart is
important. For premature infants, the Fenton or Olsen growth
curves are used.
15
For term infants, the World Health Organiza-
tion (WHO) growth curve is used until age 2, and then the Cen-
ters for Disease Control and Prevention (CDC) growth curve is
used.
16,17
Traditionally, these curves were used with percentiles.
To be more accurate in assessment, it is now recommended that
z scores be used. A z score is a statistical measure of how far a
point is from the mean. Using percentiles, the only way to
describe a very low-weight child was to state that he or she was
below the third percentile. This could either describe a child just
barely below the third or a child severely below the third percen-
tile. With z scores, these points are given numeric values and
they can be compared from one measurement to the next.
18,19
Another useful measurement in the assessment of pediatric
malnutrition is mid–upper arm circumference (MUAC).
2,20–22
The WHO has MUAC standards from 6–59 months,
20
and
other references are available for older children and adults.
21
MUAC has been shown to correlate with BMI in children.
22
More information on assessment of pediatric malnutrition is
available elsewhere.
20
Physical exam. Along with weight status, nutrition-focused
physical exam findings should include assessment of skin
Table 1. Selected Clinical Conditions Relevant to a Patient’s Ability to Tolerate EN.
3–8
Prematurity in the neonate results in immature GI motility and risk of developing necrotizing enterocolitis.
Trauma and critically ill patients may have altered metabolism and varying needs during the different phases of illness.
Critically ill patients with traumatic brain injury have a higher frequency of GI disorders, such as gastroparesis and subsequent
feeding intolerance.
Diabetes and certain neurological conditions place patients at risk for gastroparesis and poor EN tolerance.
Chronic obstructive pulmonary disease predisposes patients to muscle atrophy and weight loss related to chronic inflammation,
increased metabolism, and other physiologic derangements.
Ventilator-dependent respiratory failure may affect decision of formula selection and concentration.
Altered GI anatomy resulting from small bowel resection, bariatric surgery, other GI surgery, or fistula affects decision making
about feeding route and formula selection.
Altered GI anatomy also poses a risk of anastomotic leak, malabsorption leading to diarrhea, and subsequent loss of nutrients, which
may result in metabolic derangements.
Renal failure affects the patient’s ability to tolerate fluid volume and electrolytes.
Hemodynamic instability may preclude the safe initiation of EN in the critical care patient.
Cancer and ongoing treatments such as high-dose radiation to the head/neck may result in inflammation of the esophagus with dysphagia.
Dysmotility conditions associated with gastroschisis or scleroderma may impact ability to tolerate EN.
Neuromuscular diseases such amyotrophic lateral sclerosis can result in dysphagia
EN, enteral nutrition; GI, gastrointestinal.

20 Journal of Parenteral and Enteral Nutrition 41(1)
integrity, fluid accumulation or deficit, muscle and fat loss, and
functional status. Handgrip strength is a predictive indicator of
postoperative complications, hospital length of stay and read-
mission, and physical status. Physical therapists may offer a
valuable assessment of physical function. Muscle function cor-
relates well and reacts quickly to changes in nutrition status. In
pediatric patients, developmental status and risk of aspiration
with oral intake should be evaluated.
23,24
Assessment of malnutrition and nutrition needs. Malnutrition
is also associated with longer hospital length of stay, higher
cost of hospitalization, increased risk for readmission, and
increased mortality.
25
Indeed, it is the third most common rea-
son for 30-day readmission among selected surgical patients.
26
With up to 50% of hospitalized patients reported to be mal-
nourished, it is a critical factor to consider during nutrition
assessment.
13,26
In the neonatal population, data show that
improvement in growth and neurodevelopment outcomes are
correlated with better nutrition intake.
27
Although there is no universally accepted approach to the
diagnosis and documentation of malnutrition, standardized
protocols should be put in place to assess each patient’s anthro-
pometric and laboratory data, previous and current food/nutri-
ent/fluid intake, and functional recommendations from the
Academy of Nutrition and Dietetics and the American Society
for Parenteral and Enteral Nutrition.
1
The use of a standardized
approach to identify and treat malnutrition can lead to cost-
effective patient-centered nutrition support therapy.
28
Question 1.2. What are the required elements of the EN
therapy recommendation and where are they to be
documented?
Practice Recommendations
1. Include these required elements in the EN therapy
recommendations as listed below. These data will be
consistent with the elements of the subsequent EN
prescription.
a. Indication for EN therapy and rationale
b. Enteral formula name, concentration if appropriate
(such as kcal/oz in pediatrics), and modular
component names as appropriate
c. Enteral access device, including tip placement
d. Volume per feeding or total volume per day
e. Initial rate, goal rate, and advancement schedule
f. Rationale for recommending a specialized enteral
formula or suggesting a change (as applicable)
g. The specific method of feeding (such as
continuous, intermittent gravity, or bolus) is
specified, as well as the feeding route and access
device
h. Schedule and amount of routine water flushes, if
applicable
i. The daily nutrients to be provided at goal,
including total daily volume of formula, calories,
protein, and free water. Grams of carbohydrate
may be useful in patients with diabetes. Record
nutrients per kilogram of body weight such as
grams of protein and kcal per kilogram.
j. Monitoring required to identify adverse events,
such as refeeding syndrome, GI intolerance, or
tube malposition, as early as possible
2. Recommend modular products, such as additional
protein, fiber, and other supplements along with
administration schedule, as appropriate. Note final
kcal/oz for pediatric patients.
3. Include additional elements of feeding protocols, such
as keeping the head of the bed (HOB) elevated, oral
care/decontamination or holding the feeding for
abdominal distention, vomiting, new or worsening
hypotension, or other indications of intolerance.
4. Specify baseline or routine laboratory markers and
monitoring.
5. Document the recommendations of nutrition support
clinician in the EHR that allows access for all
healthcare providers.
Rationale
The success of EN relies on the expertise of nutrition support
clinicians. The most current Standards of Practice for nutrition
support clinicians outline the level of professional responsibil-
ity and clinical expertise required or expected of these health-
care professionals.
29–33
Important elements of the EN
recommendation made by the nutrition clinician address the
monitoring of biochemical data, anthropometrics, nutrient
needs, enteral access, EN tolerance, and other indicators.
33
Communication and implementation of the EN recommenda-
tions are essential for successful nutrition intervention and
may impact outcomes in terms of desired weight gain,
improved markers of nutrition status, and reduced hospital
length of stay.
34,35
Providing recommendations for use of feed-
ing protocols has resulted in increased number of days on EN,
more total EN volume and calories delivered, and improved
GI tolerance.
34
Documenting the nutrition assessment and recommenda-
tions in the EHR allows for quicker communication and imple-
mentation of the recommendations, as well as better
accessibility and legibility than other documentation methods,
such as paper charts.
36
A standardized uniform and complete
recommendation will allow the prescribers and the rest of the
healthcare team accessing the EHR to fully understand the
nutrition recommendations and rationale.
Question 1.3. What is the most effective way to
communicate the recommendation for EN therapy to
the licensed prescriber?

Boullata et al 21
Practice Recommendations
1. Communicate the recommendation in a standardized,
timely, and accurate manner.
2. Use the EHR system to communicate the nutrition
assessment and nutrition recommendations to the
licensed prescriber.
3. Consider a facility policy that allows registered
dietitians or other nutrition clinicians to order medical
nutrition therapy, per state regulations and institutional
privileges.
4. Program the EHR so the nutrition assessment and EN
recommendation flow directly into the order entry
section of the EHR for prescribers to review and accept.
5. Verbally communicate the recommendations to the
prescriber in addition to permanent documentation
through the EHR.
Rationale
Effective 2-way communication between nutrition support
clinicians, the prescriber, and the primary care team is critical
in order to implement nutrition support therapy recommenda-
tions in a timely manner. Where state regulation and facility
policy grant EN order-writing privileges for the registered
dietitian or other nutrition clinician, the plan may be reviewed
and implemented immediately.
34,35,37
In these cases, the plan is
always communicated with the healthcare provider, who has
ultimate responsibility for the patient’s care. This communica-
tion is safest and most direct when the nutrition plan is docu-
mented in a central location, such as the medical section of the
EHR.
38
Current methods of communication among healthcare pro-
viders regarding EN orders vary from one facility to the next.
Perhaps the most easily standardized method of communication
is the EHR. Communication via this method is more accessible,
legible, and immediate than other methods and therefore may
result in improved outcomes, including improved EN volume
and calorie provision.
36,38
Whenever possible, additional com-
munication between the recommending clinician and the pre-
scribing physician is encouraged. Open dialogue between 2 or
more people improves communication and information sharing
in the context of healthcare.
39–44
In-person discussion is consid-
ered more effective than other methods of communication
(such as telephone calls, e-mail, or text messaging) to reinforce
the assessment and recommendations provided in the EHR (or
paper chart if still in use). In the inpatient setting, in-person
communication can occur during interdisciplinary patient care
rounds, but follow-up written documentation is important.
39–44
Topics for Future Research
Multidisciplinary use of nutrition-focused physical
examination indicators
Integration of nutrition assessment parameters in the
EHR
EHR support in calculating nutritional parameters, fluid
requirements, nutrition risk assessment tools, etc
Methods of communicating nutrition assessment and
recommendations and outcomes
National standardization of EHRs
Nutrition informatics, translational research,
telemedicine
References
1. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy
of Nutrition and Dietetics and American Society for Parenteral and Enteral
Nutrition: characteristics recommended for the identification and docu-
mentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral
Nutr. 2012;36(3):275-283.
2. Becker P. Carney LN, Corkins MR, et al. Consensus statement of the
Academy of Nutrition and Dietetics/American Society for Parenteral
and Enteral Nutrition: indicators recommended for the identification and
documentation of pediatric malnutrition (undernutrition). Nutr Clin Pract.
2015;30:147-161.
3. Pinto TF, Rocha R, Paula CA, de Jesus RP. Tolerance to enteral
nutrition therapy in traumatic brain injury patients. Brain Inj.
2012;26(9):1113-1117.
4. Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American
College of Gastroenterology. Clinical guideline: management of gastro-
paresis. Am J Gastroenterol. 2013;108(1):18-38.
5. DeBellis HF, Fetterman JW. Enteral nutrition in the chronic obstructive
pulmonary disease (COPD) patient. Pharm Pract. 2012;25(6):583-585.
6. Miller KR, Kiraly LN, Lowen CC, Martindale RG, McClave SA.
“CAN WE FEED?” A mnemonic to merge nutrition and intensive
care assessment of the critically ill patient. JPEN J Parenter Enteral
Nutr. 2011;35(5):643-659.
7. Peev MP, Yeh DD, Quraishi S, et al. Causes and consequences of inter-
rupted enteral nutrition: a prospective observational study in critically ill
surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27.
8. Jadcherla SR, Gupta A, Stoner E, Fernandez S, Caniano D, Rudolph CD.
Neuromotor markers of esophageal motility in feeding intolerance infants
with gastroschisis. J Pediatr Gastroenterol Nutr. 2008;47(2):158-164.
9. Bittencourt AF, Martins JR, Logullo L, et al. Constipation is more fre-
quent than diarrhea in patients fed exclusively by enteral nutrition: results
of an observational study. Nutr Clin Pract. 2012;27(4):533-539.
10. Banh L. Serum proteins as markers of nutrition: what are we treating?
Pract Gastroenterol. 2006;43:46-64.
11. Mueller CM, Compher C, Druyan ME. A.S.P.E.N. clinical guidelines:
nutrition screening, assessment, and intervention in adults. JPEN J
Parenter Enteral Nutr. 2011;35(1):16-24.
12. Fuhrman MP, Charney P, Mueller CM. Hepatic proteins in nutrition
assessment. J Am Diet Assoc. 2004;104:1258-1264.
13. Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, Daniels L.
Malnutrition and its impact on cost of hospitalization, length of stay, read-
mission and 3-year mortality. Clin Nutr. 2012;31(3):345-350.
14. Fry DE, Pine M, Jones BL, Meimban RJ. Patient characteristics and the
occurrence of never events. Arch Surg. 2010;145(2):148-151.
15. World Health Organization. WHO Child Growth Standards. Geneva,
Switzerland: World Health Organization; 2008. http://www.who.int/
childgrowth/en/. Accessed May 16, 2016.
16. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the
Fenton growth chart for preterm infants. BMC Pediatrics. 2013;13:59.
17. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for
the United States: methods and development. National Center for Health
Statistics. Vital Health Stat. 2002;11:246.

22 Journal of Parenteral and Enteral Nutrition 41(1)
18. WHO Multicentre Growth Reference Study Group. WHO Child Growth
Standards: Growth Velocity Based on Weight, Length and Head
Circumference: Methods and Development. Geneva: World Health
Organization; 2009.
19. Centers for Disease Control and Prevention. Growth charts. 2000 CDC
growth charts for the United States. September 9, 2010. http://www.cdc.
gov/growthcharts/. Accessed May 16, 2016.
20. De Onis M, Yip R, Mei Z. The development of MUAC for age refer-
ence data recommended by a WHO expert committee. Bull World Health
Organ. 1997;75:11-18.
21. Frisancho AR. New norms of upper limb fat and muscle areas for assess-
ment of nutritional status. Am J Clin Nutr. 1981;34:2540-2545.
22. Schweizer J, Gerver WJ. Mid upper arm circumference is a reliable pre-
dictor of body mass index in healthy Dutch children [abstract]. J Pediatr
Gastroenterol Nutr. 2005;40:695.
23. Lawson CM, Daley BJ, Sams VG, Martindale R, Kudsk KA, Miller
KR. Factors that impact patient outcome: nutrition assessment. JPEN J
Parenter Enteral Nutr. 2013;37:30S-38S.
24. Norman K, et al. Hand grip strength: outcome predictor and marker of
nutritional status. Clin Nutr. 2011 Apr;30(2):135-42.
25. Corkins MR, Guenter P, DiMaria-Ghalili RA, Jensen GL, Malone A,
Miller S. Malnutrition Diagnoses in Hospitalized Patients: United States,
2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186-195.
26. Kassin MT, Stobäus N, Gonzalez MC, Schulzke JD, Pirlich M. Risk fac-
tors for 30-day hospital readmission among general surgery patients. J Am
Coll Surg. 2012;215(3):322-330.
27. Ehrenkranz RA. Nutrition, growth, and clinical outcomes. World Rev Nutr
Diet. 2014;110:11-26.
28. Hofer M, Pozzi A, Joray M, et al. Safe refeeding management of anorexia
nervosa inpatients: an evidence-based protocol. Nutrition. 2014;30: 524-530.
29. Brantley SL, Russell MK, Mogensen KM, et al. American Society for
Parenteral and Enteral Nutrition and Academy of Nutrition and Dietetics
revised 2014 standards of practice and standards of professional perfor-
mance for registered dietitian nutritionists (competent, proficient, and
expert) in nutrition support. Nutr Clin Pract. 2014;29:792-828.
30. Mascarenhas MR, August DA, DeLegge MH, et al. Standards of practice
for nutrition support physicians. Nutr Clin Pract. 2012;27(2):295-299.
31. Tucker A, Ybarra J, Bingham A, et al. A.S.P.E.N. standards of practice for
nutrition support pharmacists. Nutr Clin Pract. 2015;30:139-146.
32. DiMaria-Ghalili RA, Gilbert K, Lord L, et al. Standards of nutrition care
practice and professional performance for nutrition support and generalist
nurses. Nutr Clin Pract. 2016;31:527-547.
33. Heyland DK, Cahill NE, Daliwal R, et al. Impact of enteral feeding proto-
cols on enteral nutrition delivery: results from a multicenter observational
study. JPEN J Parenter Enteral Nutr. 2010;34(6):675-684.
34. Braga JM, Hunt A, Pope J, Molaison E. Implementation of dietitian rec-
ommendations for enteral nutrition results in improved outcomes. J Am
Diet Assoc. 2006;106(2):281-284.
35. Roberts SR. Improving patient outcomes through registered dietitian order
writing. Nutr Clin Pract. 2013;28(5):556-565.
36. Vanek VW. Providing nutrition support in the electronic health record era:
the good, the bad, and the ugly. Nutr Clin Pract. 2012;27(6):718-737.
37. Krenkel JA, St. Jeor ST. The quality of RD written communication with
physicians and the relationship to clinical practice factors. J Acad Nutr
Diet. 2007;107(8)(suppl 3):A-72.
38. Tappenden KA, Quatrara B, Parkhurst M, Malone AM, Fanjiang G,
Ziegler TR. Critical role of nutrition in improving quality of care: an inter-
disciplinary call to action to address adult hospital malnutrition. JPEN J
Parenter Enteral Nutr. 2013;37(4):482-497.
39. Gausvik C, Lautar A, Miller L, Pallerla H, Schlaudecker J. Structured
nursing communication on interdisciplinary acute care teams improves
perceptions of safety, efficiency, understanding of care plan and teamwork
as well as job satisfaction. J Multidiscip Health. 2015;8:33-37.
40. Berber R, Pappas Y, Khoo M, et al. A new approach to managing patients
with problematic metal hip implants: the use of an Internet enhanced mul-
tidisciplinary team meeting: AAOS exhibit selection. J Bone Joint Surg
Am. 2015;97(4):e20.
41. Lamb BW, Jalil RT, Sevdalis N, Vincent C, Green JS. Strategies to
improve the efficiency and utility of multidisciplinary team meetings in
urology cancer care: a survey study. BMC Health Serv Res. 2014;8;14:377.
42. Gilardi S, Guglielmetti C, Pravettoni G. Interprofessional team dynamics
and information flow management in emergency departments. J Adv Nurs.
2014;70(6):1299-1309.
43. Lawn S, Delany T, Sweet L, Battersby M, Skinner T. Barriers and enablers
to good communication and information-sharing practices in care planning
for chronic condition management. Aust J Prim Health. 2015;21(1):84-89.
44. Nahikian-Nelms M. Interprofessional simulation: strengthening ties to
increase communication and improving patient care. J Acad Nutr Diet.
2013;113(9)(suppl 3):A67.
Section 2. Prescribing and Communicating
the Enteral Nutrition Order
Background
In comparison with the greater risks associated with PN, the pre-
scription of EN may seem benign, but patient harm can occur
when EN practice recommendations are not followed. Adverse
events related to EN have been reported at each step of the EN
process. Examples of these events include enteral feeding tube
malposition or misconnection, EN formula contamination, and
bronchopulmonary aspiration.
1
Therefore, patient safety is a fun-
damental consideration in the EN prescribing process. Prescribers
of EN need in-depth knowledge of protein and energy require-
ments, electrolyte and fluid balance, acid-base homeostasis, and
GI anatomy and function. Prescribers of EN must also be knowl-
edgeable in proper indications and contraindications to EN,
proper care and selection of EADs intended for gastric or small
bowel placement, and potential complications related to EN.
2–5
Currently, EN orders may be inconsistently worded and
executed due to the individualized prescribing habits of clini-
cians, variance between institutions, and inadequate prescriber
education. Furthermore, many organizations still sanction pre-
scribing EN via telephone, verbal, or handwritten orders. The
use of standardized electronic EN orders can help address
problems of incomplete, ambiguous, or incorrect EN orders.
This section will provide guidance for healthcare organizations
when developing policies and procedures to safely prescribe
and communicate the EN order.
Question 2.1. How can the approach to prescribing EN
be standardized to reduce EN-related errors?
Practice Recommendations
1. Use a standardized approach for prescribing EN to
minimize complications associated with incomplete or
ambiguous EN orders.
2. Develop and implement policies and procedures that
address all aspects of the EN order process and
competency assessments for healthcare professionals
involved in the prescription of EN.

Boullata et al 23
3. Apply a standardized model of prescribing for safe EN
practice, with each organization using the insight of
their prescribers to determine how best to apply the
model. Consider including EN prescribing in ongoing
professional practice evaluation (OPPE) and focused
professional practice evaluation (FPPE).
4. Incorporate interdisciplinary teams as available within
the organization, allowing each member to address
relevant issues as it relates to the EN process.
5. Develop and implement a process for the primary
healthcare team to assess, document, and communicate
the therapeutic goals and monitoring of EN therapy.
Following the process, the primary healthcare team can:
a. Evaluate the patient to assess that EN
administration is safe and indicated.
b. Confirm that the patient has an appropriately
placed EAD that is appropriate in regards to
current clinical status.
c. Review the nutrition assessment and nutrition
recommendations as documented by nutrition
support clinicians (see Section 1).
6. Describe specific methods of communication to be used
among physicians, advanced practice providers, dietitians,
pharmacists, and nurses involved with the prescription,
order review, administration, and monitoring of EN.
7. Involve clinicians specializing in nutrition support in
the design of a standardized EN order process that will
meet the needs of the organization’s specific patient
population.
a. Prescribe EN for all patients using standardized
electronic EN orders (eg, computerized provider
order entry [CPOE] systems).
b. When CPOE systems are unavailable, prescribe
EN with a standardized order template using an
editable electronic document, saved as a PDF,
which will remain part of the EHR.
c. Avoid handwritten, telephone, and verbal EN orders
because of the potential for transcription errors.
d. Design electronic EN order sets with clear
instructions that are easily understood by all
healthcare professionals involved in the
prescription of EN.
8. Design a transitional EN order template that assists
with the transition from acute care to long-term care or
home care settings (see Section 11). Using a well-
designed standardized template will facilitate
communication of the following:
a. Patient identifiers, previous EN formula and water
flushes, delivery site and access device, and
administration method and rate
b. Previously trended laboratory values and clinical
assessments relevant to EN tolerance
c. Contingency plans for transition to oral feedings
or PN as circumstances may dictate
Rationale
Organizations need proper, accurate documentation of nutrition
interventions that is available to all members of the healthcare
team. This documentation can promote effective 2-way commu-
nication between prescribers of EN and those reviewing EN
orders and subsequently monitoring the patient regarding appro-
priate energy and protein delivery, changes in therapy, medica-
tion interactions, EN tolerance, and other pertinent information.
The implementation of a standardized EN ordering process
that includes an electronic order template can eliminate the
possibility for inappropriate EN orders due to omissions, tran-
scription errors, or illegible documentation. When all elements
of the EN order are included during electronic prescription, the
risk for errors related to verbal order clarification and tran-
scription can be lessened. Standardized EN orders can also
guide all EN prescribers within an institution to use the same
terminology when referencing EN.
6,7
Other advantages of stan-
dardized orders can include preventing incomplete orders and
improving efficiency for the prescriber and enhancing patient
safety. When all elements of the EN order are included during
electronic prescription, there is a reduced risk for errors.
6
The adoption of EHRs can give nutrition support profes-
sionals an opportunity to implement standardized EN order
processes. In a recent national survey of hospital pharmacy
directors by the American Society of Health-System
Pharmacists, 80.9% of hospitals that responded were using
CPOEs for general medication orders.
8
However, the degree of
customization within electronic systems is low. Nutrition sup-
port clinicians will need to work closely with information tech-
nology personnel (who can in turn reach out to vendor and
application architects as needed) to request adequate decision
support capability and proper documentation for those pre-
scribing EN. In a survey of the American Society for Parenteral
and Enteral Nutrition’s membership regarding the safety and
efficacy of nutrition documentation and nutrition-related
ordering processes, Vanek
9
found that nutrition support practi-
tioners do not highly rate their institutions’ EHR systems and
concluded that the growing adoption of EHRs and CPOE sys-
tems offers nutrition support practitioners the opportunity to
ensure that nutrition and nutrition support content within their
system is adequate and safe. Ammenwerth et al
10
conducted a
systematic review to determine the effect of CPOE systems on
general medication error and adverse drug events. Within the
systematic review, 25 out of 27 studies addressed medication
errors. Of those 25, 23 studies showed a relative risk reduction
for medication errors of 13% to 99% after implementation of
CPOE. Ammenwerth and colleagues also concluded that a
transparent culture of safety within healthcare systems can
increase proper reporting of medication errors, which will pro-
vide better data for future research.
11
Documentation of nutrition interventions should be avail-
able to all members of the healthcare team. Proper documenta-
tion allows prescribers of EN to communicate EN tolerance,

24 Journal of Parenteral and Enteral Nutrition 41(1)
EAD status, changes in therapy, and any other pertinent infor-
mation to the rest of the healthcare team. This documentation
should allow for communication between prescribers of EN and
those reviewing EN orders for appropriate energy, protein, and
fluid delivery; medication interactions; and EN tolerance.
11
Malone et al
12
reported a case of a 65-year-old woman who
was supposed to receive EN through a gastrostomy tube and
fluid and electrolyte replacement via central venous catheter.
However, she inadvertently received 160 mL of EN through her
central line when it was mistaken for the gastrostomy tube. She
subsequently required hydration, diuretic therapy, and prophy-
lactic antibiotics, after which she recovered and was discharged
from the acute care setting 8 days later. This case is an example
of errors among healthcare providers in a patient with multiple
access devices. Electronic EN orders can specifically indicate
proper EN administration directions and may help eliminate
errors related to orders that could expose patients to harm.
10
The use of a complete EN order specifically designed to pre-
scribe EN for home or transitional use will promote the continu-
ity of a patient’s care. The EN regimen can be optimized while
the patient is in an inpatient setting, and the nutrition support
clinician can reassess nutrition needs before discharge. A com-
plete EN transition order will also allow the primary outpatient
clinician to take over patient care and determine the appropriate
frequency of laboratory monitoring, reassessment of nutrition
needs, and confirmation of tube placement. EN transition orders
can also assist with self-management of home enteral feedings
in those who do not receive skilled nursing services. A complete
order for discharge can allow for adequate education to be pro-
vided to patients being discharged to home with EN.
13
Overall, a standardized approach to the EN prescription
process that is administratively supported by the organization
can ensure patient safety, assist the entire healthcare team, and
help provide cost-effective nutrition therapy. Nutrition support
clinicians must be engaged and held accountable for the devel-
opment and implementation of policies and procedures related
to the EN prescription process.
Questions 2.2 and 2.3. What are the critical (required)
elements for a complete EN order? What are the
supplementary (auxiliary) elements to the EN order
that may improve patient safety?
Practice Recommendations
1. Include the following critical elements in the standardized
electronic EN order template (Figures 2 and 3):
a. Patient information
i. Identify patients by the following: patient
name, date of birth/age, and medical record
number.
ii. Transmit patient-specific information
relevant to the electronic EN order such as
height/length and dosing weight and allergies
(eg, food, medication).
b. EN formula name
i. Describe EN primarily via descriptive
generic names (eg, “standard,” “high
protein”) to minimize confusion for
prescribers. The product trade name could
also be included along with the
organizationally defined generic term. For
pediatric patients, add final kcal/oz.
c. Delivery site (route) and EAD
i. Include the administration route in the EN
order based on the enteral tube’s distal tip
position (gastric or small bowel).
ii. The specific EAD to be used (eg, nasogastric
[NG], orogastric, gastrostomy, nasojejunal,
orojejunal, jejunostomy, or gastro-
jejunostomy).
d. Administration method and rate
i. Include the specific method of administration
in the EN order (eg, continuous, bolus,
intermittent feedings).
ii. Define the volume and rate of administration
of EN for each method of administration.
iii. Order sets that include advancement can be
populated with the standard advancement
and held, to be released each day after the
clinician examines the patient and reviews
orders with the team.
2. Develop nurse-driven EN protocols for volume-based
feeding as per institutional policy.
a. Include the volume and frequency of water
flushes.
b. Provide suggested methods to advance the volume
and/or rate toward goal.
3. Create and implement policies and procedures that
promote all elements of the EN order to be completed
whenever the EN order is modified or reordered.
4. Design electronic order sets with elements that promote
patient safety.
a. Use required fields within the EN order to prevent
submission of the order until it is complete.
b. Use menus to facilitate standardization of EN
prescribing.
5. When EN is reordered, require that prescribers take
accountability for the proper monitoring of the
patient’s clinical condition, EN tolerance, and
metabolic status.
a. Monitor patients with newly initiated EN, newly
placed permanent EADs, critically ill patients,
patients at risk for refeeding syndrome, patients
with poor glycemic control, or patients recovering
from recent surgery as they will require more
frequent monitoring.
6. Design and implement policies and procedures that
address supplementary EN orders within the CPOE.
See Figure 4.

Boullata et al 25
a. Confirm that the initial enteral feeding tube
position is correct via proper radiographic imaging
that visualizes the entire enteral feeding tube. The
exception to this may be in pediatric and neonatal
patients who require multiple tube placements due
to the x-ray exposure (see Section 4).
b. Establish proper EAD flushing in supplementary
orders (see Section 7). Develop protocols that call
for proper flushing before and after medication
administration, during continuous feedings,
before and after intermittent feedings, and before
and after gastric residual volume (GRV)
measurements.
c. Address reassessment of the appropriateness of
HOB elevation and ongoing monitoring for EN
tolerance in policies and procedures.
d. Integrate EAD care and assessment into policies
and procedures to assist with infection prevention
INPATIENT ENTERAL NUTRITION ORDER
Patient Name: ____________
Room Number:___________
Medical Record Number: _____________ Dosing Weight (kg): ______________________
Date of Birth: __________
Allergies: ______________
Total Energy kcal/day
_____________
Total Protein g/day
____________
Total Carbohydrate g/day
______________
Total Fat g/day_______________
Total Fluid mL/day___________
ENTERAL NUTRITION FORMULA
□ Standard
□ Standard High protein
□ Standard High Calorie
□ Fiber Containing
□ Carbohydrate controlled
□ Elemental include peptide-based
□ Immune modulating
□ Renal – low electrolytes
DELIVERY SITE (ROUTE AND ACCESS)
Route:
□ Gastric
□ Small bowel
Access:
□ Nasogastric
□ Nasoduodenal
□ Nasojejunal
□ Orogastric
□ Oroduodenal
□ Orojejunal
□ Gastrostomy
□ Jejunostomy
□ Transgastric G/J tube
ADMINISTRATION (Method and Rate)
Method:
□ Continuous
□ Intermittent
□ Bolus
Rate:
□ Initial ____________________________ mL/h
□ Advance by _________ mL/h every _________ h to goal of __________ mL/h
□ Initial ________ mL feeding over ________ min _________ times daily
□ Advance by _________ mL each day to goal of _________ mL feeding
over __________ min _________ times daily
□ Initial ________ mL bolus over _________ min _________ times daily
□ Advance by _________ mL each day to goal of ___________ mL bolus
over __________ min __________ times daily
OTHER
□ Flush feeding tube with _____________ mL of water every __________ hours (minimum of 30 mL per flush)
□ Elevate head of bed 30–45 degrees
Figure 2. Enteral nutrition order template (specific content can be customized per institution). G/J, gastrojejunostomy.

26 Journal of Parenteral and Enteral Nutrition 41(1)
and allow for proper intervention if a complication
occurs.
e. Ongoing monitoring includes laboratory
monitoring, measurement of intake and output,
weight measurements, physical assessment, and
GI tolerance.
f. Identify the specific product for modular therapies
along with the proper prescribed amounts and
administration schedule.
g. State specific amounts of additional macronutrients
per day with orders for modular nutrition therapies
(eg, 12 g protein powder per day) along with
directions for proper reconstitution and
administration.
7. Make consultation to the nutrition support team or
clinical nutrition service available for prescribers.
8. Determine the duration (time limits) of the EN order
before it has to be renewed.
Rationale
The development of clearly defined policies and procedures
regarding the required elements of the EN order helps the facil-
ity ensure that the orders are complete throughout the EN pro-
cess and that the right patient receives the right product, in the
right amount, via the right route at the right time. It is recom-
mended that the essential elements of the EN order are made
available for viewing by all healthcare professionals via proper
electronic documentation in the EHR. Critical elements for a
complete EN order must be addressed through a CPOE order or
editable electronic document before supplementary elements
can be acknowledged.
14
In a prospective study, Armada et al
15
evaluated the effect of the implementation of the CPOE system
on the incidence of prescription errors and found that prescrip-
tion errors decreased significantly from the error rate for hand-
written of 44.8% to an error rate of 0.8% after CPOE
implementation (P < .001). This prospective study demonstrates
□ Start feedings of Human Breast Milk (HBM) at 1 mL q3h via NG tube (15 mL/kg/day, @ 10 kcal/kg).
□ Continue for 3 days for trophic feedings.
□ Increase feedings by 1 mL q3h per day on day 4, 5, and 6 of the feeding protocol until feeds on day 7 are at 75 mL/kg (5 mL q3h).
□ On day 8 continue same feeding volume and begin fortification of feeds to 24 kcal/oz using human milk fortifier, 1 packet to 25 mL of
human milk.
□ On day 8 and thereafter the advancement continues at 1 mL q3h until the total volume is 160 mL/kg or 11 mL q3h on day 14. This will
provide 160 mL/kg, @128 kcal/kg, @ 4.5 g/kg protein.
□ Do not routinely check gastric residuals.
□ Do not routinely flush NG tube.
□ Continue daily weights.
□ Obtain length measurements using (length board) and head circumference measurements (taking the average of three measurements) weekly.
□ After reaching full-volume feedings, add vitamin D (400 International Units) and evaluate the baby for the need for additional elemental iron.
Figure 3. Example of neonatal enteral nutrition feeding protocol. NG, nasogastric.
SUPPLEMENTARY ORDERS
Auxiliary Orders:
□ Assess gastric residual volume (GRV) every 6 hours or before each bolus/intermittent feeding
If GRV > 500 mL hold feeding for 2 hours and recheck GRV. If GRV recheck < 500 mL, restart feeding
□ May give appropriate medications via enteral feeding tube, follow each medication by at least 15 mL water flush before and after medication as
volume allowed (do not mix medications together or with EN formula)
□ Consult Nutrition Support Team or Nutrition Support Clinician
Monitoring:
□ Observe for signs of EN intolerance (include signs and symptoms of intolerance) every ____________ hours
□ Enteral feeding tube site care and assessment every ____________ hours
□ Obtain body weight every day, or every ______________ days
□ Strict fluid volume Ins/Outs
□ Capillary blood glucose: per institutional protocol
Laboratory Orders:
□ Comprehensive Metabolic Panel every day or every ________ days
□ Serum Magnesium every day or every _________ days
□ Serum Phosphorus every day or every _________ days
Figure 4. Suggested enteral nutrition (EN) supplementary orders (specific content can be customized per institution).

Boullata et al 27
the impact that healthcare technology can have on patient safety,
and it helps nutrition support professionals justify the impor-
tance of nutrition-based software integration.
15
It is important
when developing electronic EN ordering documents that institu-
tion specific and patient population customization is permitted
(Figures 2 and 3).
The appropriate initiation and advancement of an EN regi-
men depend on the patient condition as well as the administra-
tion method and EAD type. Continuous EN administration via
enteral feeding pump with small-volume, frequent water flushes
is preferred in the critically ill, those at risk for intolerance, and
for small bowel feedings. Directions for continuous EN admin-
istration identify the proper initial administration rate and can
contain supplementary orders addressing timing of rate
advancement to goal infusion volume. Bolus and intermittent
methods of EN administration via syringe, regulated drip
enteral feeding bag, or enteral feeding pump are preferred in
patients who have proven tolerance with continuous EN admin-
istration and those who will transition out of the acute care set-
ting with EN. Directions for bolus and intermittent EN
administration document the proper number of feedings per day
along with initial proper volume of EN administration rate and
volume and frequency of water flushes. Bolus and intermittent
feeding orders can also contain supplementary orders that give
directions for volume advancement and goal EN volume.
The implementation of enteral feeding protocols may
improve energy, protein, and fluid delivery to ICU patients who
experience interruptions in EN delivery due to unavoidable pro-
cedures (reintubation/extubation, bedside procedures involving
the GI tract or airway, and imaging studies).
16,17
The administra-
tion of large volumes of EN to compensate for EN that was
missed during procedures can place patients at risk for intoler-
ance of EN.
18–21
If enteral feeding protocols are going to be
implemented, healthcare organizations should utilize multidisci-
plinary teams to determine if these protocols are beneficial for
that institution’s patient population and how to build this into the
order entry process. See Figure 3 for an infant EN protocol.
Supplementary orders (see Figure 4) assist with adequate
energy and protein delivery, maintain patient safety, and assist
clinical staff with therapeutic monitoring of EN therapy.
Although supplementary orders are not essential, they comple-
ment the EN order with additional guidance to better communi-
cate and standardize EN for a patient. Supplemental orders will
be based on institutional policies that advocate for the proper
care of the enterally fed patient within the practice variations at
each organization. These orders can also permit prescribers to
consult an institution’s nutrition support service to assist with
management of EN. Supplementary orders address the use of
adjunct modular therapies, which can allow clinicians to
enhance macronutrient contents of an EN prescription.
Critical and supplementary elements of the EN order facili-
tate proper and safe EN prescription and administration.
Nutrition support clinicians can help institutions determine and
develop any supplementary orders that would benefit their
patient population. Continued review of institutional policies
and procedures along with national clinical guidelines and
practice recommendations will allow institutions to continue to
improve the EN process.
Question 2.4. What is the safest way to describe EN
formulas?
Practice Recommendations
1. Set policies and procedures on how EN formulas will
be described throughout the healthcare organization,
including in electronic order sets, patient-specific EN
labels, and all other references to EN (eg, for product
inventory, purchasing, healthcare provider
documentation).
2. Describe EN primarily via descriptive generic names
(eg, “standard,” “high protein”) to minimize confusion
for prescribers. The product trade name could also be
included along with the organizationally defined
generic term.
3. Develop a patient-specific EN label template to reflect
all the critical elements of the EN order.
Rationale
The EN prescription should be a patient-specific therapy that is
prescribed, reviewed, prepared, and administered, with a pro-
cess optimized for patient safety. The use of CPOE has been
shown to reduce the opportunity for medication errors due to
illegible orders, transcription errors, and prescriber error.
22
The
use of electronic order sets in CPOE can positively assist pre-
scribers when obtaining patient-specific and EN formula infor-
mation. However, with constantly evolving medication trade
names and EN formula brand names and product labeling, there
is opportunity for transcription error when acting on an EN
order, especially if it is handwritten. EN formula-specific infor-
mation should be easily accessible to prescribers to allow for
the delivery of adequate protein and energy, electrolytes, and
fluid and to ensure proper EN formula prescription. Disease-
specific formulas should be selected using clinical judgment
with knowledgeable clinicians weighing efficacy, tolerance,
cost, and clinical evidence (from randomized clinical trials).
14
Determine descriptive generic names to be used to describe
EN formulas throughout the entire healthcare system. The use
of generic names to describe EN is encouraged because health-
care organizations often change EN formularies and because
EN formularies will vary among the acute, chronic, and home
care settings. Brand names for EN can be confused when other
formula or medications have similar names. When institutions
change EN formularies, it is important that clinicians have easy
access to formulary changes and a “formulary card” or “con-
version chart” with new EN formulas, old EN formulas, and
modular products available. For example, an EN formula that

28 Journal of Parenteral and Enteral Nutrition 41(1)
contains nonhydrolyzed macronutrients that is intended for
those with normal digestive function can be generically identi-
fied as “standard.” An EN formula that contains hydrolyzed
macronutrients, which could be used for those with malabsorp-
tive disorders, can be generically identified as “peptide-based”
or “elemental.” An EN formula that contains a higher percent-
age of calories from fat along with a higher fiber content to
assist with glycemic control can be generically identified as
“carbohydrate controlled.”
Develop policies and procedures regarding patient-specific
EN formula labels that can be affixed to EN formula adminis-
tration containers. Develop patient-specific EN formula labels
that contain all of the elements in the same sequence as the
original EN order. Determine if patient-specific EN formula
labels present all nutrients or only macronutrients and select
micronutrients.
Question 2.5. How often should the EN order be
reviewed for renewal in the acute care, chronic care,
and home care settings?
Practice Recommendations
1. Determine an institution-specific or organization-
specific policy for the frequency of EN order review
and renewal based on the level of care provided by the
institution (acute care vs subacute care vs long-term
care vs home care).
2. Complete all elements of the EN order when the EN
order is modified or reordered.
3. Review orders daily in conjunction with monitoring
daily in unstable patients (eg, critically ill patients,
postsurgical patients, patients with poor glycemic
control, patients with unstable fluid and electrolyte
status, and patients at risk for refeeding syndrome).
4. Review orders daily for neonatology and critical
pediatric patients. Stable pediatric patients may need
less frequent review.
5. Reduce monitoring of EN orders to every 2–7 days (1–3
times per week) in stable adult hospitalized patients.
6. Monitor patients in the long-term care or home setting
who have demonstrated to be stable on an EN
prescription with no signs of intolerance every 1–4
weeks. Less frequent review and reordering may be
appropriate in select patients on long-term EN in
keeping with regulatory requirements.
Rationale
Even though EN may seem to be a benign therapy, there are
complications and adverse events related to the EN process.
Policies and procedures addressing the timeframe for the
renewal of the EN order will help facilities have the best EN
order system based on the patient’s current condition.
By monitoring the patient and reviewing the EN orders at
appropriate frequencies, clinicians can provide nutrition sup-
port that is safe, able to detect any clinical or metabolic compli-
cations, and assess the extent to which nutrition goals have been
reached. Unlike PN, which may require frequent adjustments,
the EN regimen may not require therapeutic interventions as
frequently. Often, the EN order is best reviewed and renewed
when a patient changes levels of care or when the patient on EN
is discharged to home or a long-term care facility.
Existing literature does not address the ideal frequency for
reviewing EN orders. Therefore, practitioners must rely on
expert clinical experience and consensus opinion to provide
clinical practice guidelines. The ideal timeframe for EN order
review and renewal may vary based on the healthcare setting
and the acuity of the patient population. Patients newly initiated
on EN will need more frequent monitoring than those whose
tolerance of EN has been established. Special attention is also
given to high-risk patients, such as those who are clinically
unstable (eg, patients with preexisting metabolic abnormalities,
critically ill patients, or postoperative patients) and those at risk
for refeeding syndrome. The frequency of order review usually
decreases as patients stabilize and transition to lower levels of
care. In long-term care settings, time intervals between order
renewals may be subject to regulatory standards.
Each healthcare organization can establish its own policy
regarding the frequency of the EN order review and renewal.
Clinicians with expertise in the area of nutrition support, pref-
erably from multiple disciplines, are key players to engage in
policy development. To ensure patient safety and assess the
effectiveness of nutrition interventions, organizations will
want to monitor compliance with policies.
Question 2.6. What educational programs and
systematic changes can be implemented to prescribers
of EN to improve EN ordering and reduce errors?
Practice Recommendations
1. Provide education regarding safe practices for EN
prescribing and monitoring to all clinicians that
prescribe EN.
2. Provide ongoing rigorous education about safe EN
prescribing practices to improve communication and
monitoring. Educational initiatives can include
healthcare team in-services, pocket cards, and regular
audits with reporting results at institutional quality
improvement meetings.
3. Integrate education regarding safety in EN into the
core curriculum for healthcare students and trainees. A
multidisciplinary team of clinicians with expertise in
the area of nutrition support can conduct this education.
4. Provide in-depth and rigorous educational content on
safety issues to all clinicians who will care for patients
receiving EN in the acute, chronic, and home care

Boullata et al 29
settings and those who are training to specialize in
nutrition support care.
5. Evaluate or design a physical environment for EN
prescribing by assessing needs that may affect the
performance of EN prescribers to safely communicate
the EN order for transcription, interpretation, and
review in the following 5 factors outlined by the United
States Pharmacopeial Convention, USP General
Chapter <1066>:
a. Characteristics of the individual prescriber can
vary in responses to physical environment.
Therefore, adaptation to the physical environment
to meet individual needs will optimize accuracy
of all prescribers of EN.
b. Tasks performed and workloads: Prescribers
presented with large workloads often find
workarounds and overrides that could place
patient safety at risk.
c. Tools and technology used to perform tasks: With
the constant evolution of technology within
healthcare, the tools and technologies implemented
in healthcare systems must be user-friendly, easily
accessible, and optimized to each institution’s needs.
d. Compliance of the physical environment in
relation to USP General Chapter <1066>: Sensory
interference from noise, light, interruptions, or
poorly constructed work environments can
adversely impact the ability of clinicians to safely
prescribe EN.
e. Organizational support: Offer support that helps
address new and ongoing concerns related to the
safe communication and transcription of the EN
order.
6. Avoid verbal and telephone prescriptions except for
communication between prescriber and nutrition
support clinician to clarify the EN order that may result
in order revision.
Rationale
Research is limited regarding whether educational programs
about safe EN prescribing practices affect patient outcomes.
However, studies have shown that patient care with multidisci-
plinary teams increases communication among healthcare pro-
fessionals, which in turn contributes to higher rates of patient
safety,
23
and this finding suggests that educational techniques
that improve communication among members of the EN team
may be warranted. Further research on the impact of the educa-
tion of EN prescribers on the incidence of EN-related errors
and inappropriate prescribing is needed.
23
The implementation of education programs has been asso-
ciated with safer practices for prescribing medication.
24
Elements of safe EN prescribing are appropriate topics for the
core didactic curricula in professional programs (medical,
pharmacy, advanced practice nursing, nutrition, and physician
assistants). Safe practices for prescribing EN can also be inte-
grated into the clinical training for professional programs, resi-
dencies, and specialty/fellowship programs for those who may
be involved in the prescribing of EN.
7
The process of prescribing EN requires an environment that
is productive for each prescriber of EN and an environment
that is designed with consideration of the following: prescriber
characteristics, workload of prescribers and those implement-
ing orders, technology available, and organizational support.
The October 2010 bulletin by the USP, titled “Physical
Environments That Promote Safe Medication Use,” establishes
work environment standards to reduce the risk of medication
errors. This bulletin gives nutrition support professionals a
resource to incorporate safe EN prescribing practices into poli-
cies and procedures for clinical practice.
25
Question 2.7. What are the essential elements of safe
communication and transcription of the EN order?
Practice Recommendations
1. Create policies and procedures that minimize the need
for order transcription, therefore limiting transcription
errors and increasing safe communication within the
EN order process.
2. Use EHR communication technology to avoid
transcription during the EN order process.
3. Institute and follow policies and procedures to
encourage that transcribed orders are independently
double-checked for completeness and accuracy before
EN review and preparation.
a. Whenever possible, avoid multiple transcriptions
of EN order data.
b. If manual data transcription is completely
unavoidable, document any transcribed data that
undergoes a double-check process and make it
available for quality improvement audits.
4. Review and compare EN orders to the most current
recommendations when reassessing patients. Whenever
there are unexplained discrepancies between the order
and the recommendations, communicate with the
healthcare team according to institutional policies to
ensure that recommendations were understood.
5. Develop protocols/algorithms to serve as com-
munication tools and guides to safe EN practice for the
healthcare organization. These may include guidance
about the following:
a. Initiation of EN prior to completion of nutrition
assessment by the dietitian or other nutrition
support clinician
b. Approach to feeding through various EADs
c. Water-flushing protocols, especially if using
automated systems

30 Journal of Parenteral and Enteral Nutrition 41(1)
d. Medications that can be given via EADs and if
tube feedings need to be held (see Section 8)
Rationale
An incomplete order, missing data, required transcription
step, or inadequate verbal communication between prescrib-
ers and those ultimately implementing the EN order increases
the risk for errors that can adversely affect patient care. The
use of technology can assist with the provision of safe EN
therapy. The development of standardized EN order forms
can facilitate consistent prescription of complete EN orders
without the need for interpretation or transcription. As EN
prescribers adopt the use of standardized orders, the process
of standardized independent double-checks with stepwise
checklists becomes easier as orders are prescribed and com-
municated to other staff in a consistent manner. To have an
effective process, 2 clinicians must independently review the
EN order prior to preparation and labeling. The use of inde-
pendent double-checks should not be overused as to cause
fatigue for healthcare providers, but they should assist with
addressing potential breakdowns found in the EN process.
Independent double-checks must be used in conjunction
with other safety measures, and education should be pro-
vided to reiterate the importance of independent double-
checks to healthcare staff.
26
Multidisciplinary teams can assist with the facilitation of
open communication between members of the healthcare dis-
ciplines. Teamwork between disciplines can also improve rela-
tionships between departments within the healthcare system,
and this communication can lead disciplines to better under-
stand the demand on other disciplines. This open communica-
tion can improve the EN process by increasing team members’
knowledge and facilitate learning about problems. The rela-
tionships built with the use of multidisciplinary teams can also
ease the communication between providers when clarifying or
optimizing an EN regimen. Communication between teams
can also lead to identification of a problem, finding the root
cause of the problem, and development of a team-based multi-
disciplinary action plan.
27
Evidence-based EN protocols/algorithms developed by
nutrition support professionals serve as a guide for safe, stan-
dardized EN practice and communication. Their use has been
shown to minimize the use of inappropriate EN, increase EN
days, increase the percentage of prescribed calories delivered,
and reduce hospital stays and mortality. In order for protocols/
algorithms to be used in practice, ongoing and rigorous educa-
tion and monitoring are needed.
Topics for Future Research
Documentation of errors related to EN prescribing
The impact of electronic EN orders on the accuracy,
monitoring, and safety of EN therapy
The effect of standardized orders on adequate protein
and energy delivery
Error rates related to incomplete, ambiguous, or
incorrect EN orders
Error rates associated with use of standardized EN
orders vs error rates with the use of telephone, verbal, or
handwritten EN orders
Outcomes research regarding how the frequency of
monitoring of EN orders affects the achievement of
patient safety and nutrition goals
The impact of education programs and annual
competency assessment on errors related to EN ordering
and patient safety measures
The use of a standardized EN home transition order
form in the continuity of care for patients discharged
home with EN
References
1. Guenter P, Hicks RW, Simmons D. Enteral feeding misconnections: an
update. Nutr Clin Pract. 2009;24(3):325-334.
2. Brantley SL, Russell MK, Mogensen KM, et al. American Society for
Parenteral and Enteral Nutrition and Academy of Nutrition and Dietetics
revised 2014 standards of practice and standards of professional perfor-
mance for registered dietitian nutritionists (competent, proficient, and
expert) in nutrition support. Nutr Clin Pract. 2014;29:792-828.
3. Mascarenhas MR, August DA, DeLegge MH. Standards of practice for
nutrition support physicians. Nutr Clin Pract. 2012;27(2):295-299.
4. Tucker A, Ybarra J, Bingham A, et al. A.S.P.E.N. standards of practice for
nutrition support pharmacists. Nutr Clin Pract. 2015;30:139-146.
5. DiMaria-Ghalili RA, Gilbert K, Lord L, et al. Standards of nutrition care
practice and professional performance for nutrition support and generalist
nurses. Nutr Clin Pract. 2016;31:527-547.
6. Hsu C, Chou CL, Chen TJ, et al. Physicians failed to write flawless pre-
scriptions when computerized physician order entry system crashed. Clin
Ther. 2015;37(5):1076-1080.
7. Guenter P, Boullata JI, Ayers P, et al. Standardized competencies for
parenteral nutrition prescribing: the American Society for Parenteral and
Enteral Nutrition model. Nutr Clin Pract. 2015;30(4):570-576.
8. Pedersen CA, Schneider PJ, Scheckelhoff DJ. ASHP national survey
of pharmacy practice in hospital settings: dispensing and administra-
tion—2014. Am J Health System Pharm. 2015;72(13):1119-1137.
9. Vanek VW. Providing nutrition support in the electronic health record era:
the good, the bad, and the ugly. Nutr Clin Pract. 2012;27(6):718-737.
10. Ammenwerth E, Schnell-Inderst P, Machan C, et al. The effect of elec-
tronic prescribing on medication errors and adverse drug events: a system-
atic review. J Am Med Inform Assoc. 2008;15(5):585-600.
11. Emami S, Hamishehkar H, Mashayekhi, S, et al. Errors of oral medication
administration in a patient with enteral feeding tube. J Res Pharm Pract.
2012;1(1):37-40.
12. Malone M, Aftahi S, Howard L. Inadvertent intravenous administra-
tion of an elemental enteral nutrition formula. Ann Pharmacother.
1993;27(10):1187-1189.
13. Kozeniecki M, Fritzshall R. Enteral nutrition for adults in the hospital set-
ting. Nutr Clin Pract. 2015;30(5):634-651.
14. Brown B, Roehl K, Betz M. Enteral nutrition formula selection: current
evidence and implications for practice. Nutr Clin Pract. 2015;30(1):72-85.
15. Armada ER, Villamanan E, Lopez de-Sa E, et al. Computerized physician
order entry in the cardiac intensive care unit: effects on prescription errors
and workflow conditions. J Crit Care. 2014;29(2):188-193.
16. Heyland DK, Dhaliwal R, Lemieux M, Wang M, Day AG. Implementing
the PEPuP protocol in critical care units in Canada: results of a multi-

Boullata et al 31
center, quality improvement study. JPEN J Parenter Enteral Nutr.
2015;39:698-706.
17. Taylor B, Brody R, Denmark R, Southard R, Byham-Gray L. Improving
enteral delivery through the adoption of the “feed early enteral diet ade-
quately for maximum effect (FEED ME)” protocol in a surgical trauma
ICU: a quality improvement review. Nutr Clin Pract. 2014;29:639-648.
18. Ventura AM, Waitzerg DL. Enteral nutrition protocols for critically ill
patients: are they necessary? Nutr Clin Pract. 2015;30(3):351-362.
19. Heyland DK, Cahill NE, Daliwal R, et al. Impact of enteral feeding proto-
cols on enteral nutrition delivery: results from a multicenter observational
study. JPEN J Parenter Enteral Nutr. 2010;34(6):675-684.
20. Gungabissoon U, Hacquoil K, Baines C, et al. Prevalence, risk factors,
clinical consequences, and treatment of enteral feed intolerance during
critical illness. JPEN J Parenter Enteral Nutr. 2015;39(4):441-448.
21. Peev MP, Yeh DD, Quarishi SA, et al. Causes and consequences of inter-
rupted enteral nutrition: a prospective observational study in critically ill
surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27.
22. Hilmas E, Peoples JD. Parenteral nutrition prescribing processes using
computerized prescriber order entry: opportunities to improve safety.
JPEN J Parenter Enteral Nutr. 2012;36(suppl 2):32-35.
23. Dingley C, Daugherty K, Derieg MK, et al. Improving patient safety
through provider communication strategy enhancements. In: Henriksen
K, Battles JB, Keyes MA, et al, eds. Advances in Patient Safety: New
Directions and Alternative Approaches. Rockville, MD: Agency for
Healthcare Research and Quality; 2008. http://www.ncbi.nlm.nih.gov/
books/NBK43663/
24. Boullata J, Gilbert K, Sacks G, et al. A.S.P.E.N. clinical guidelines: par-
enteral nutrition ordering, order review, compounding, labeling, and dis-
pensing. JPEN J Parenter Enteral Nutr. 2014;38(3):334-377.
25. USP Chapter <1066>. Physical Environments That Promote Safe
Medication Use. Washington, DC: The United States Pharmacopeial
Convention; 2010.
26. ISMP. Independent double checks: undervalued and misused: selective
use of this strategy can play an important role in medication safety. ISMP
Medication Safety Alert! June 13, 2013. http://www.ismp.org/newsletters/
acutecare/issue.asp?dt=20130613. Accessed June 26, 2013.
27. Hughes RG. Tools and strategies for quality improvement and patient
safety. In: Hughes RG, ed. Patient Safety and Quality: An Evidence Based
Handbook for Nurses. Rockville, MD: Agency for Healthcare Research
and Quality; 2008. AHRQ Publication No. 08-0043.
Section 3. Review of the Enteral Nutrition
Order
Background
A dedicated review of the EN order by a nutrition support pro-
fessional ensures that the order contains all the critical elements
for a complete EN order and that it meets the specific patient’s
energy, protein, micronutrient, and fluid needs. This review is
conducted independently from the EN recommendation and the
EN prescription. Safety issues in the EN order review can
involve the correct patient identification; the appropriateness of
the prescribed EN formula for the patient; dosing, administra-
tion, and monitoring instructions; free water flushes; the EAD;
concurrent medications and potential drug-nutrient interactions;
the EN infusion site; and the effect of EN on the patient’s elec-
trolyte, acid-base, and fluid balances. Healthcare organizations
must have policies and procedures that address the EN review
process for nutrition support professionals and determine how
interventions will be communicated to the primary team.
Question 3.1. What are the best mechanisms and
practices for independent EN order review for safe
and optimal EN preparation and delivery?
Practice Recommendations
1. Develop and implement policies and procedures
at the healthcare organizational level that address
the independent review of the EN order by a
knowledgeable healthcare provider and the documen-
tation of the review process for safety and clinical
audits.
2. Prescribe EN using standardized electronic order
templates (ie, CPOE system) that transmit the complete
EN order.
a. In the absence of a CPOE system with standardized
templates, prescribe EN with a standardized order
template that is maintained as an editable
electronic document with each patient-specific
order saved as a pdf in the EHR, and implement
best practices to avoid transcription errors from
handwritten or telephone orders.
b. Enter EN order data in a standardized format,
and transmit any supplemental orders in standard
units. Include order instructions that are clear to
those reviewing or administering EN.
c. Make nutrition assessment and nutrition
recommendations available in the EHR.
3. Include the EN order in the patient’s electronic
medication profile to allow a pharmacist to review the
EN order and patient medication profile. The
pharmacist will assess:
a. The appropriateness of the medication route of
administration
b. The compatibility of medication with enteral
formulas
c. Methods to optimize the medication regimen
4. Evaluate the following elements as part of the
clinician’s independent review of the EN order:
a. Patient allergies
b. Proper dosing weight
c. Current clinical status and nutrition needs
d. Indication for therapy
e. Appropriate energy, protein, micronutrient, and
fluid delivery
5. Develop clear policies and procedures for the
healthcare organization to address the clarification of
EN orders if any of the following occur:
a. Order elements are missing.
b. Clinical dosing does not meet recommendations.
c. Administration is inconsistent with guidelines or
may be associated with incompatibilities.
6. Document any order clarification or change to the EN
order within the facilities’ EHR or, in the absence of

32 Journal of Parenteral and Enteral Nutrition 41(1)
EHR, document any clarification to the EN order in the
permanent record.
7. Do not use abbreviations, symbols, or dose designations
that appear on The Joint Commission’s Official “Do
Not Use List” or on the Institute for Safe Medication
Practices list of Error-Prone Abbreviations, Symbols,
and Dose Designations.
8. Review the EN prescription independently whenever
there are transitions in patient care (eg, admission to a
facility, discharge from a facility, or any change in the
level of care within a facility).
9. Develop criteria at the healthcare organization level to
annually evaluate the competency of nutrition support
clinicians and pharmacists to review EN orders and to
assess associated patient laboratories, medications, and
clinical monitoring.
Rationale
The standardization of the EN order process can increase
compliance with independent double-checks and improve
patient safety at all points of the healthcare model.
1
Use of
CPOE systems along with clinical decision support software
can facilitate the review of each element critical to a complete
EN order. Ideally, a nutrition support clinician or other
knowledgeable healthcare provider (“reviewer”) will review
EN orders in an environment with minimal distractions,
appropriate lighting, and access to electronic patient and EN
formula information. For optimal review, the electronic EN
order will contain the critical elements required for a com-
plete order (eg, patient identifiers, EN formula, free water
flushes, delivery site and EAD, and administration method
and rate) as well as any supplementary orders that meet insti-
tution-specific needs related to the safe prescription of EN
and the adequate delivery of protein, calories, and fluid. If the
reviewer concludes that a critical element is omitted or a ther-
apeutic intervention would be beneficial, this reviewer must
communicate with the prescriber to reconcile missing ele-
ments or recommend clinical interventions. Any communica-
tion with the EN prescriber should be electronically
documented in the EHR.
When a patient on EN requires medications, it is advisable
to consult a pharmacist to determine whether a medication can
be safely prepared and administered via the EAD. The pharma-
cist can also review medication profiles for medications that
could cause adverse effects when administered via either a gas-
tric or small bowel EAD. Schier et al
2
reported a case of a
38-year-old woman who received crushed extended-release
antihypertensive medications via a nasogastric tube. The
instant release of medication that was intended to release over
a 24-hour period led to the patient’s death secondary to brady-
cardia and severe hypotension. This case shows how important
the review process can be to ensure proper administration of
medication via enteral feeding tube.
The inclusion of the EN order in the electronic medication
profile and the medication administration record (MAR)
enables the pharmacist to review the EN order along with med-
ications to be administered to the patient. When CPOE systems
are appropriately configured, prescribers enter orders for medi-
cations that specify the specific administration method (eg,
nasogastric feeding tube, orogastric feeding tube, small bowel
feeding tube, gastrostomy tube, jejunostomy tube, or desig-
nated port of gastrojejunostomy tube). The pharmacist can then
review the record to assess the compatibility of any medica-
tions that are to be concomitantly administered with EN and
determine the most optimal formulation of the medication to
be administered through the EAD. The pharmacist will indi-
cate whether EN is to be held for a period of time before or
after medication administration (see Section 8).
Handwritten orders can increase the risk of transcription
errors or incomplete EN orders. Bobb and colleagues
3
reviewed
prescribing errors within a 700-bed academic medical center,
and they used their findings to assist with CPOE implementa-
tion in their institution. Out of a total of 17,808 inpatient and
emergency department orders that were processed during the
week of the study, 1111 orders (6.2%) contained a prescribing
error. The most common error types identified were wrong
dose, wrong nomenclature, and wrong frequency. This group
determined that CPOE systems can improve practitioner pre-
scribing habits, and that the use of CPOE systems with clinical
decision support software in conjunction with clinical staff
involvement can mitigate most prescribing errors. The use of
detailed standardized electronic order sets can limit the number
of possible transcription errors and can promote safety by
eliminating the option to choose incorrect elements of the EN
order (eg, if jejunostomy feeding is selected, only continuous
administration options would be available for selection).
4,5
The Institute for Safe Medication Practices List of Error-
Prone Abbreviations, Symbols, and Dose Designations and The
Joint Commission’s “Do Not Use” list are important consider-
ations for healthcare organizations building electronic EN order
forms. The Joint Commission’s “Do Not Use” list currently
does not apply to EHRs including CPOE systems. However,
healthcare organizations will want to eliminate the use of these
abbreviations, symbols, or dose designations from their EHR
software so that they do not appear in standardized EN orders.
6,7
Independent reviews and procedures to double-check EN
orders require time and resources. If these requirements seem
disruptive or burdensome to practitioners, the risk of noncom-
pliance can increase. Organizations will need to allow resources
to fulfill this critical step in the EN process.
As the patient’s level of care changes, proper reconciliation
and communication of the EN order is vital to patient safety
and continuity of nutrition care. The World Health Organization
reports that up to 46% of errors occur when new orders are
written at patient admission or discharge.
8
As patients transi-
tion to different levels of care, the risk of errors related to the
EN order grows. For example, if transfer orders need to be

Boullata et al 33
printed and reconciled before a change in therapy is made, the
patient may not receive the new therapy promptly after the
transition in level of care.
Question 3.2. What are the critical elements of the EN
order that need to be transmitted to optimize a
complete review?
Practice Recommendations
1. Prescribe EN using a standardized electronic EN order
template (ie, CPOE system). In the absence of a CPOE
system, prescribe EN with a standardized order
template (can use an editable electronic document)
format (eg, using an Excel spreadsheet, with each
unique order saved as a pdf).
2. A complete EN order contains all of the following
critical elements:
a. Patient information: Include the following patient
identifiers: patient name, date of birth/age, and
medical record number.
b. Describe EN primarily via descriptive generic
names (eg, “standard,” “high protein”) to
minimize confusion for prescribers. The product
trade name could also be included along with the
organizationally defined generic term.
c. Delivery site (route) and access device
i. Identify the delivery site by the enteral
feeding tube tip position (gastric or small
bowel).
ii. Identify the specific EAD (nasogastric
feeding tube, orogastric feeding tube, small
bowel feeding tube, gastrostomy tube,
jejunostomy tube, designated port of
gastrojejunostomy tube).
d. Administration method and rate
i. Document the method of administration
(continuous, bolus, intermittent feedings).
ii. Clearly define the volume and/or rate of EN
administration for each method of
administration.
iii. Include any suggested methods to advance
the volume and/or rate toward goal.
iv. Guidelines for volume based feeding if
applicable.
v. Address the advancement of EN to
established goal along with transitions from
PN to EN, EN to diet, or hospital to home/
alternative care sites.
vi. Document instructions for water flushes,
including the solution to be used (eg, purified
water), volume, frequency, and timing, as
well as the volume to be administered in
24-hour period.
3. Use supplementary orders to assist with the care of the
EN patient and help ensure patient safety.
Supplementary orders can include:
a. Confirmation of tube position
b. Evaluation of skin
c. Assessment of tolerance
d. Specific laboratory monitoring
e. Recommendations for modular products
f. Instructions for EN preparation, including
directions for reconstitution of powder (if
indicated), shaking contents of can/container,
wiping off can with alcohol prep
g. Nutrition support consult
h. Head of bed elevation
i. Oral care/decontamination
j. GRV checks
4. If EN orders are modified, reordered, or ordered upon
hospital discharge/transfer, verify that all elements of
the EN order are completed and independently
reviewed by a nutrition support professional.
Rationale
A complete EN order will maintain patient safety while ensur-
ing adequate EN formula delivery and proper EN administra-
tion. The EN order should contain the following critical
elements: (1) patient identifiers, (2) EN formula type, (3)
delivery site and access device with identification of correct
port for infusion, and (4) administration method, EN infusion
rate or volume of EN to be infused at stated intervals, and vol-
ume of water flushes at stated intervals. The EN order should
be transmitted for review via CPOE or by an electronic edit-
able document if CPOE is not available at a healthcare institu-
tion. These electronic orders can assist with the appropriate
prescription of EN by decreasing improper EN prescription
and eliminating order transcription.
Patient information. Patient identifiers, including the patient’s
name, date of birth, and medical record number (MRN), help
ensure that the right patient receives the correct EN order. The
use of standardized electronic EN orders could eliminate the
possibility of the wrong patient receiving the EN order by
requiring the use of adequate multiple patient identifers.
9
EN formula name. The EN formula can be clearly identified in
the electronic order by a descriptive generic name and/or trade
name that is identified on the can, container, or package. For
example, an EN formula that contains nonhydrolyzed macronu-
trients that is intended for those with normal digestive function
can be generically identified as “standard.” An EN formula that
contains hydrolyzed macronutrients, which could be used for
those with malabsorptive disorders, can be generically identi-
fied as “peptide based” or “elemental.” An EN formula that
contains a higher percentage of calories from fat along with a

34 Journal of Parenteral and Enteral Nutrition 41(1)
higher fiber content to assist with glycemic control can be
generically identified as “carbohydrate controlled.”
EN delivery site, route, and access device. The EN delivery
site with correct route (gastric or small bowel) and EAD is a
critical element of the EN order. The proper identification of
delivery site and device can decrease the possibility of enteral
feeding tube misconnections. Route of administration identi-
fies gastric or small bowel tube feedings, whereas enteral
access identifies short-term devices, including nasogastric or
orogastric feeding tubes (NGT, OGT), or percutaneous devices,
including gastrostomy (G), jejunostomy (J), or gastrojejunos-
tomy (G/J) tubes. The identification of site for EN administra-
tion and medication administration can decrease the possibility
of EN administration via the wrong access when more than 1
port or access device is present.
Administration method and rate. The EN order includes the
proper method of administration and EN infusion rate. The
administration method identifies whether EN is to be adminis-
tered via pump, gravity, or bolus methods. The infusion sched-
ule lists the infusion times and initial rate or volume to be
infused per feeding. It should also include an advancement
rate/volume along with the total volume to be infused within a
24-hour period. The infusion schedule also identifies the vol-
ume and frequency for water flushes, which may change as
the EN infusion and volume change in the absence of IV flu-
ids. The EN schedule identifies whether EN is administered
via a continuous drip, intermittent drip, cyclic drip, or bolus
delivery.
Organizations can implement standardized electronic order
sets that prevent prescribers from ordering improper adminis-
tration methods for specific EADs. For example, electronic
orders for jejunostomy feedings could only allow prescribers
to order continuous feeding administration. The specificity of
choices in essential and supplementary orders can make the
EN order review process more efficient.
Supplementary orders. Supplementary orders can be devel-
oped according to each institution’s identified needs and
patient population. They can be used to assist with the advance-
ment of EN, as well as transitions from PN to EN, EN to oral
diet, or one facility to another facility/home. The care of enter-
ally fed patients is also appropriately addressed in supplemen-
tary orders. For example, HOB elevation, enteral feeding tube
care, GRV checks, and monitoring and laboratory parameters
are to be addressed by supplementary orders.
Prescribers of EN refer to available information about EN
formulas when they order EN. The use of electronic order
sets can help prescribers determine whether a base EN for-
mula will provide adequate macronutrient content for a
24-hour period or if a supplementary prescription of modular
macronutrients can help meet the patient’s needs. Patients
with fluid tolerance limits (eg, pediatric patients; patients
with renal failure or heart failure) may need augmentation of
base EN formulas with a modular macronutrient to increase
calories without additional fluid. Populations with high pro-
tein needs may need additional protein modulars, whereas
those who require protein restriction may benefit from carbo-
hydrate or fat modulars. Institutions can decide whether to
provide micronutrient, electrolyte, and water content of EN
formulas to prescribers via information boxes within the
CPOE system. The inclusion of micronutrient and electrolyte
data in the ordering system can help prescribers select EN
products for patients who have electrolyte imbalances or con-
ditions where micronutrients are either not eliminated prop-
erly or are depleted with high-volume fluid losses.
Question 3.3. What steps can be taken to evaluate EN
access, administration timing, fluid requirements,
and other critical elements related to the enterally
fed patient?
Practice Recommendations
1. Develop and institute policies and procedures at the
healthcare organizational level that define the roles and
responsibilities of each individual involved in the EN
therapy process.
2. Support a multidisciplinary committee that reviews the
healthcare organization’s policies and procedures and
analyzes errors related to the EN therapy process.
a. Develop protocols for EAD assessment and care.
b. Develop protocols regarding EN hang time and
proper labeling of the beyond use date and time
for EN formulas.
c. Develop surveillance programs to monitor and
review cases of EN formula contamination.
d. Develop protocols for proper administration of
medications through an EAD, as well as
appropriate water flushes and EAD declogging
procedures.
3. Optimize the EN prescription, administration, and
order review process with methods, technology, and
procedures that improve patient safety and decrease
opportunities for lapses in clinician adherence to
institutional policies.
4. Standardize the EN process at the healthcare
organizational level to assist with the consistent
delivery of patient care.
5. Develop EHR systems that can address the nutrition
support clinician’s concerns related to an institution’s
patient-specific population.
6. Institute policies and procedures regarding the
documentation of the assessment of EN patients and
independent double-check processes.
7. Develop protocols that incorporate checklists for each
individual in the EN therapy process.

Boullata et al 35
Rationale
The proper evaluation of the enterally fed patient can optimize
patient safety while monitoring the provision of energy, pro-
tein, fluid, and medications. Patients receiving EN may have
electrolyte abnormalities, acid-base disorders, and fluid imbal-
ances that can be potentiated with administration of calories.
Patient monitoring of the patient’s metabolic status, feeding
tolerance, and EAD placement are all essential in the delivery
of EN. When monitoring also includes surveillance of high-
risk steps within the EN process, healthcare organizations can
improve the EN process as a whole.
Enteral access device placement and maintenance. The pri-
mary healthcare team should choose an EAD after evaluating
current anatomy, clinical status, and estimated course of ther-
apy. After the EAD is selected and its initial placement is con-
firmed via radiograph, the EAD placement must be continually
reassessed. Patient movement, coughing, suctioning, emesis,
or movement of the tube within the tube securement tape/
device can cause the distal tip of a feeding tube to migrate dis-
tal or proximal to the intended site. A malpositioned EAD
could lead to gagging/emesis of EN formula aspiration and
sepsis. A malpositioned long-term EAD can lead to site leak-
age, blockage of the pylorus, and buried bumper syndrome. To
confirm that the position of the EAD has not changed, the doc-
umented EAD length of the numerical marking at the exit site
of the tube is assessed every 4 hours or before being accessed.
Continue to confirm proper placement by comparing docu-
mented length or the numerical marking at the exit site of the
EAD every 4 hours or prior to being accessed. If patient assess-
ment leads a clinician to think that the EAD has migrated or is
malpositioned, confirm the EAD placement by radiograph in
adults
10
(see Section 4 for EAD placement).
Healthcare organizations also need protocols that address
the care and maintenance of EAD sites. One protocol can out-
line the procedure for notifying the medical team if the patient
has new or increasing pain, excess leakage, redness, swelling,
induration, or bleeding from the enteral feeding tube site. Some
incisional pain is expected after an initial percutaneous EAD
insertion, but it should lessen over time. Protocols can also
address the notification of the medical team if the patient has
pain, nausea, feelings of fullness, or emesis during EN infu-
sions as these signs could indicate a malpositioned EAD.
Clinicians must follow institutional guidelines regarding dress-
ing changes and wound care. In 2010, the National Patient
Safety Agency reported the case of a patient who underwent
percutaneous endoscopic gastrostomy (PEG) placement and
had postprocedure pain and leakage from the gastrostomy site.
This patient was discharged and then readmitted 4 days later
with internal leakage. The patient died 3 weeks later secondary
to sepsis from the PEG site.
11
This case reinforces that EAD
site maintenance is important to patient care and appropriate
observation can decrease the risk of adverse events.
Question 3.4. What systems need to be in place to make
order clarifications and interventions to improve the
safety and delivery of EN?
Practice Recommendations
1. Create policies and procedures that address the proper
methods for order clarifications and clinical
interventions for EN orders.
2. Document each order clarification or clinical
intervention within the patient’s EHR.
3. Conduct regular systematic reviews of all documented
order clarifications and clinical interventions with a
multidisciplinary team and create action plans to
address any shortcomings identified within the EN
process.
4. Independently review each EN order by a clinician
whose competencies are assessed by the healthcare
organization.
Rationale
The continual systematic review of oversight in the EN process
helps identify gaps in the EN process. Many organizations
review incident/event reporting and near misses, but problems
can be substantially underreported if employees fear repercus-
sions from error reporting. Healthcare organizations that create
a culture of transparency allow employees to report errors
without fear of repercussions.
Healthcare organizations need cost-efficient methods to
identify and review medical errors. Meyer-Massetti and col-
leagues
12
performed a systematic review of 28 studies to assess
the accuracy, efficiency, and efficacy of 4 medication safety
assessment methods: incident report review, direct observa-
tion, chart review, and trigger tool. They found that each
method of identifying drug safety–related problems has dis-
tinctive advantages and disadvantages and the various methods
identify different types of safety issues. Therefore, they recom-
mend that healthcare organizations select the methods that best
fit the context and the nature of the suspected problems.
Accurate documentation of clinical interventions can pro-
vide objective data to justify clinical staffing and evaluate clini-
cal staff performance. This documentation can also demonstrate
that clinical staff takes accountability for patient care and avoids
unnecessary costs. However, the interpretation of the quality of
clinical interventions can be limited when quality improvement
measures are lacking. Rector and colleagues
13
described the
implementation of an education project to improve documenta-
tion of clinical interventions by pharmacists. They found that a
pharmacist education initiative led to increased clinical inter-
vention documentation with increased documentation of costs
avoided. This initiative led the quality improvement project to
stratify clinical intervention by their appropriateness and rein-
force a new culture for pharmacy trainers.

36 Journal of Parenteral and Enteral Nutrition 41(1)
Optimization of the independent double-check process
ensures that practitioners think critically while conducting
checks as designed. However, independent double-checks can
be overused in the healthcare industry, and the improper use of
such checks can lead to safety concerns, especially if checks
are inconsistent or if clinicians become noncompliant.
Standardization of the independent double-check process using
checklists can reduce inconsistencies in the process, and a
review of the process can help identify reasons for noncompli-
ance or other problems. When coupled with other error reduc-
tion strategies, the use of properly implemented double-check
processes can prevent errors from reaching patients.
1
Topics for Future Research
The effect of EN ordering via CPOE or editable
electronic document on EN-related error rates
Comparison of errors associated with the use of
standardized EN order sets vs errors related to the
transcription of handwritten orders
The patient safety impact of pharmacists reviewing the
EN order with a patient’s medication profile to identify
medication interventions
Documentation of EN errors related to transitions in
level of care
Documentation of errors related to the misconnection
of EADs
The error-related consequences of standardizing the EN
order process
The use of systematic reviews to identify gaps in the
EN process
References
1. Institute for Safe Medication Practices. Independent double checks:
undervalued and misused. ISMP Medication Safety Alert! June 12, 2013.
https://www.ismp.org/newsletters/acutecare/showarticle.aspx?id=51.
Accessed August 8, 2015.
2. Schier JG, Howland MA, Hoffman RS, Nelson LS. Fatality from admin-
istration of labetalol and crushed extended-release nifedipine. Ann
Phamacother. 2003;37:1420-1423.
3. Bobb A, Gleason K, Husch M, et al. The epidemiology of prescribing
errors: the potential impact of computerized prescriber order entry. Arch
Intern Med. 2001;164(7):785-792.
4. Bankhead R, Boullata J, Brantley S, et al. Enteral nutrition practice recom-
mendations. JPEN J Parenter Enteral Nutr. 2009;33(2):122-167.
5. Institute for Safe Medication Practices. 1,000-Fold overdoses can occur,
particularly in neonates, by transposing mcg and mg. ISMP Medication
Safety Alert! September 6, 2007. https://www.ismp.org/newsletters/
acutecare/articles/20070906.asp. Accessed August 8, 2015.
6. Institute for Safe Medication Practices. ISMP’s List of Error-Prone
Abbreviations, Symbols, and Dose Designations. 2013. https://www.
ismp.org/tools/errorproneabbreviations.pdf. Accessed August 10, 2015.
7. The Joint Commission. Official “Do Not Use” List. March 2009. http://www.
jointcommission.org/assets/1/18/dnu_list.pdf. Accessed August 10, 2015.
8. WHO Collaborating Centre for Patient Safety Solutions. Assuring medi-
cation accuracy at transitions in care. Patient Safety Solutions. May 2007.
http://www.who.int/patientsafety/solutions/patientsafety/PS-Solution6.
pdf. Accessed August 8, 2015.
9. Institute for Safe Medication Practices. Oops, sorry, wrong patient! A
patient verification process is needed everywhere, not just at the bedside.
ISMP Medication Safety Alert! March 10, 2011. https://www.ismp.org/
newsletters/acutecare/articles/20110310.asp. Accessed November 20,
2015.
10. Simons SR, Abdallah LM. Bedside assessment of enteral tube placement:
aligning practice with evidence. Am J Nurs. 2012;112(2):40-46.
11. Malhi H, Thompson R. PEG tubes: dealing with complications. Nursing
Times. 2014;110(45):18-21.
12. Meyer-Massetti C, Cheng CM, Schwapach DL, et al. Systematic review
of medication safety assessment methods. Am J Health Syst Pharm.
2011;68(3):227-240.
13. Rector KB, Veverka A, Evans SK. Improving pharmacist documentation
of clinical interventions through focused education. Am J Health Syst
Pharm. 2014;71(15):1303-1310.
Section 4. Enteral Access
Background
The selection of the EAD can greatly affect the success of EN.
The optimal device and location (gastric vs small bowel) must
be determined as placement of any enteral access device
entails associated risks. If patients with an EAD are trans-
ferred to a facility without complete documentation, the
receiving facility, whether acute care, long-term care, or home
care agency, will need to confirm the type and placement of
that feeding tube prior to initiating EN. The practice recom-
mendations in this section help guide that facility or agency to
confirm the EAD type and placement prior to starting feedings
and avoid feeding through an EAD that may no longer be at
the appropriate distal site.
Complications following EAD placement can include
misplacement, which is when the tip of the EAD is placed in
an anatomical position not intended for the proper adminis-
tration of EN. EAD displacement is when the device tip
later migrates or is inadvertently moved to an anatomic posi-
tion not intended for the proper position of the device. Proper
EAD placement and maintenance help prevent aspiration of
EN, dumping syndrome, and other adverse outcomes.
Although risk of complications cannot be completely elimi-
nated, minimizing placement errors reduces the complica-
tion rate and improves patient outcomes.
Question 4.1. What are the critical components to
consider when selecting an EAD for a patient?
Practice Recommendations
1. Select an EAD based on patient-specific factors (eg, GI
anatomy, GI function, expected duration of EN).
2. Place a short-term nasoenteric or oroenteric EAD in
patients who require EN for up to approximately 4–6
weeks in duration.
3. Place a long-term EAD in patients who require EN for
longer than 4–6 weeks.

Boullata et al 37
Rationale
The selection of an EAD requires an evaluation of the patient’s
disease state, GI anatomy (taking into account past surgeries),
gastric and intestinal motility and function, and the estimated
length of therapy. The healthcare team decides whether to
place the distal tip of the EAD in the stomach or in the small
bowel. In general, gastric access is appropriate for patients
with a functional stomach free of delayed gastric emptying,
obstruction, or fistula. Small bowel feedings are most appro-
priate for patients with gastric outlet obstruction, severe gas-
troparesis, and in those with known reflux and aspiration of
gastric contents. Patients who need simultaneous gastric
decompression with small bowel feedings can be best accom-
modated by a dual-lumen gastrojejunal EAD.
EADs inserted via nasal and oral routes. EADs inserted via
the nasal and oral routes are usually intended for short-term use
(no more than 4–6 weeks) in the hospitalized patient. However,
there may be situations when use of a nasogastric access in the
outpatient setting is appropriate. Some patients, particularly
pediatric patients in the home, are able to self-place a nasogas-
tric tube as part of their own care.
EADs for long-term access. The decision concerning place-
ment of EADs for long-term EN depends on the estimated
length of therapy, the long-term goals, the patient’s disposi-
tion, and the special needs of the patient and caregivers. The
use of gastrostomy tubes (balloon and nonballoon tubes) has
become routine practice worldwide and is currently the
method of choice for medium-term and long-term enteral
feeding.
1
Two studies of adult patients with persistent dyspha-
gia due to neurological disease randomly assigned patients to
feedings via NG or PEG tube placement.
2,3
These studies
found that the patients with PEG tubes had gained more
weight and missed fewer feedings. The patients fed by NG
tube received significantly less because of tube difficulties
compared with the PEG patients, who had no such difficul-
ties.
2,3
One of the studies allowed patients with an NG tube to
cross over to a PEG tube if they had repeated tube difficulties
(usually displacement), and, consequently, only 1 of 19
patients had an NG tube in place for 4 weeks.
3
At the end of
the study, the last patient with an NG tube opted for a PEG
tube, stating that the NG tube was cosmetically unacceptable.
Concerns for pediatric patients. In the pediatric literature,
commonly accepted criteria for EN intervention depend on the
clinical condition of the patient.
4–6
EN support is considered
after other aggressive oral interventions have been tried. Pedi-
atric patients who meet the criteria for EN include:
Children with insufficient oral intake, particularly
children older than 1 year who are unable to meet
60%–80% of individual requirements for 5 days and
children younger than 1 year who are unable to meet
60%–80% of individual requirements for 3 days
Children who meet the criteria for failure to thrive,
wasting, and stunting
EN is also appropriate in a disabled child whose total feeding
time is more than 4–6 hours per day. EN can also be an option
when diet modification is used as a treatment of a disease (eg,
Crohn’s disease), food intolerance, and metabolic disorders.
7
Specific indications for feeding tubes in pediatric patients
include cystic fibrosis, neurological impairment, oral/head
and neck tumors, chronic liver disease, trauma, and extensive
burns.
4
Contraindications to EAD placement. The choice of EAD
needs to take into account contraindications to the placement
of the device. These can be divided into systemic and mechani-
cal reasons and may be relative or absolute. Systemic contrain-
dications are those where the overall condition of the patient
precludes feeding tube placement. Mechanical ones are those
where specific local conditions such as hepatomegaly or previ-
ous abdominal surgery preclude safe placement of the EAD. In
some cases, the condition may be corrected. Absolute contrain-
dications include mechanical obstruction of the GI tract (unless
the procedure is indicated solely for decompression), active
peritonitis, uncorrectable coagulopathy, or bowel ischemia.
8
Traumatic injuries to the head, face, and neck region as well as
recent transphenoidal surgery may preclude a nasally placed
EAD. A number of other conditions represent relative contrain-
dications to enteral access, such as recent GI bleeding, hemo-
dynamic instability, ascites, respiratory compromise, and
certain anatomic alterations.
8
Question 4.2. What steps are recommended to confirm
placement of a preexisting EAD prior to initiating EN?
Practice Recommendations
1. Develop a policy at the healthcare organizational level
to confirm the EAD type and placement prior to EN
initiation.
2. Assess the patient and caregiver knowledge about the
tube, such as agency or facility where the tube was
placed, insertion date, where the patient was transferred
from, and what type of tube and previous care and
feeding orders were provided to the patient or caregiver.
3. Communicate with staff from the transferring
institution, facility, or agency to obtain as much
information as possible on the EAD type, tip position,
and need for ongoing replacement and documentation.
4. Confirm type of EAD and tube placement via the
accepted methods of tube verification (see Section 4,
question 4 for methods used in adult patient and
question 5 on pediatric patients).

38 Journal of Parenteral and Enteral Nutrition 41(1)
5. Document the confirmation process and findings in the
patient’s health record.
6. Encourage transferring agencies to communicate the
full information about EAD type, insertion date, and
placement upon transfer.
Rationale
Adequate and timely transfer of information between care set-
tings during transitions in care is imperative for the safe care of
patients.
9
A percutaneously positioned tube in a GI tract that
has not fully matured may be displaced prior to or during trans-
fer, particularly if the tube is inadequately secured. If the dis-
placement is not identified, this complication may lead to
intraperitoneal administration of EN. Incomplete or incorrect
communication of the EN tube type and placement during
patient transfer may delay the administration of adequate and
appropriate nutrition. Poor communication during transitions
of care may also lead to hospital readmissions and emergency
department visits that may have been preventable.
10
The EAD type, placement, and requirements for ongoing
replacement need to be communicated in the available medi-
cal record and clinical information. Clear descriptions in
plain language without ambiguous abbreviations will mini-
mize misinterpretation and error. Ideally, this documentation
is provided by the transferring agency to the new facility
prior to discharge,
10,11
and the enteral prescription and regi-
men are transferred to the accepting care team via standard
electronic information systems that are accessible to all
healthcare providers and suppliers associated with the
patient.
11
Use of these systems may improve communication;
however, they may not be universally available or accessible.
If this information must be communicated by telephone, the
nutrition support provider at the new facility should repeat it
back to ensure that it is received and interpreted correctly.
Feeding tube information, such as brand, type, tube tip posi-
tion, need for ongoing replacement, French size, and length
(if applicable), is also verified at this time.
12
Question 4.3. What steps can be taken to enhance the
safety of bedside nasoenteric tube placement?
Practice Recommendations
1. Develop organizational policies to outline who is qualified
to place a nasoenteric tube, under what circumstances,
and with what supervision or competencies.
2. Assess patients prior to tube placement for potential
contraindications, identification of high-risk patients
for misplacement, or if bedside placement is medically
appropriate.
3. Actively assess patient tolerance during tube placement.
4. Educate and assess competencies for all clinicians
involved in tube placement.
Rationale
Addressing safety measures designed to enhance the safety of
bedside blind insertion of feeding tubes before and during
tube insertion is critical as this this is where the most serious,
potentially life-threatening adverse events occur.
13,14
This is
especially needed when considering that numerous disci-
plines, with varying degrees of training, commonly place
these tubes today. The importance of training and competency
assessment of all clinicians involved in tube insertion should
be clearly delineated.
13–16
Patient assessment prior to tube insertion is essential to
preventing placement-related injury. This could include iden-
tification of patients at high risk for pulmonary misplace-
ment; recognizing contraindications to nasal passage of tubes,
including recent history of transphenoidal surgery or basilar
skull fracture (ethmoid, sphenoid, or occipital bones); evalu-
ation of bleeding risk, including coagulation values and safe
limit cutoffs; recent bleeding from esophageal varices; time
since banding; and so on. The presence of anatomical factors
that can lead to perforation should also be part of the assess-
ment: hiatal hernia or Zenker’s diverticulum and previous
bariatric surgery. Not all patients are candidates for bedside
insertion, and fluoroscopic or endoscopic placement may
provide a safer choice for tube placement.
14
Alternate bedside methods of placement are available
and include electromagnetic placement device (EMPD), use
of carbon dioxide (CO
2
) sensing, and direct visualization
using a tube with a camera. These techniques are described
below.
13–16
Development of institutional policies and proce-
dures for placement and ongoing competency assessment is
crucial. One institution temporarily stopped placement of
tubes by untrained personnel until a quality improvement
program could be put into place.
14
It is important to docu-
ment the size and manufacturer/model of the tube once it is
placed. The diameter plays an important role in types of for-
mula and medications that can be infused, and internal
diameter can change depending on the device material and
model.
Question 4.4. What is the best way to confirm accurate
EAD placement in ADULT PATIENTS?
Practice Recommendations
1. Obtain radiographic confirmation for any blindly
placed short-term EAD to demonstrate that it is
properly positioned in the GI tract prior to its initial
use for administering feedings and medications in
adult patients.
2. When attempting to insert a short-term feeding tube,
obtain a tube aspirate for appearance and pH
measurement. The appearance and pH are likely
dependent on location.

Boullata et al 39
3. Do not rely on the auscultatory method alone to
differentiate between gastric and respiratory placement
or between gastric and small bowel placement.
4. Mark the exit site of a feeding tube at the time of the
initial placement and document either the incremental
marking on the tube or the external length of the tube
in the medical record.
5. Evaluate whether the incremental marking or external
tube length changes, and, if a change is observed, use
other bedside tests such as visualization and pH testing
of tube aspirate to help determine if the tube has
become dislocated. If in doubt, obtain a radiograph to
determine tube location.
6. For long-term feeding tubes, document tube type, tip
location, and external markings in the medical record
and in follow-up examinations.
7. Avoid use of catheters or tubes not intended for use as
EADs, such as urinary or GI drainage tubes, which
usually are without an external anchoring device. Use
of such tubes may lead to enteral misconnection as
well as tube inward migration, which can potentially
cause obstruction of the gastric pylorus or small bowel.
8. Avoid administration of feedings, fluids, or medications
through the EAD until correct position has been
confirmed.
Rationale
The patency and placement of an EAD should be confirmed
before using it for feeding or medication administration. Proper
radiographic imaging is recommended to confirm the position
of any blindly placed enteral feeding tube. Healthcare profes-
sionals cannot rely on auscultatory methods to differentiate
between gastric and bronchopulmonary tube placement
because auscultatory methods cannot distinguish tubes improp-
erly placed in the lung or coiled in the esophagus from properly
positioned tubes.
17
Nasal or oral insertion of a short-term EAD is often per-
formed at the bedside. Nasojejunal tubes may be placed blindly
or with the assistance of endoscopy, fluoroscopy, electromag-
netic, carbon dioxide sensing (capnography), or direct camera
visualization devices. Studies have demonstrated that errors in
blindly placed NG tubes are not uncommon.
14,18–20
Sorokin and
Gottlieb
14
reported a 1.3%–2.4% incidence of misplacement of
a tube in 2000 NG tube insertions into adults. Of the misplaced
devices, 28% resulted in pulmonary complications, with 2 of
these misplacements culminating in death.
Confirmation that the newly inserted EAD is correctly posi-
tioned is mandatory before feedings or medications are admin-
istered. A variety of bedside tests to determine tube placement
are used with varying degrees of accuracy. Usually bedside
detection methods serve as precursors to radiographic confir-
mation, as they may serve to decrease the number of radio-
graphs needed to a single one.
8
For a blindly inserted EAD, the
gold standard for confirming correct placement is a properly
obtained and interpreted radiograph that visualizes the entire
course of the tube.
14,21–23
Confirmation is usually provided through imaging, which
can add significant cost and time to EAD placement. Recent
adjuncts have been developed, including the use of carbon
dioxide or pH sensors to confirm intubation of the stomach
rather than the pulmonary tree.
24
Sensitivity and specificity of
those 2 methods have been reported in one trial as high as 86%
and 99%, respectively.
25
Newer technology provides the clini-
cian with multiple options in confirming tube location prior to
the initiation of enteral feeding. A multicenter study compared
the use of an electromagnetic placement device (EMPD) for
placement and tube tip confirmation to standard x-ray. Of the
194 patients in this study, only 1 had data showing discrepan-
cies between the original EMPD verification and the final
abdominal radiograph interpretation, providing a 99.5% agree-
ment.
26
Other recent studies and a literature review demon-
strated similar conclusions,
27,28
while 2 more recent papers
point out the potential risk of eliminating x-ray confirmation
with inexperienced operators.
29,30
A more recent innovation is a disposable feeding tube with
an integrated real-time imaging system to visually aid in the
placement of small-bore feeding tubes. This technology
method features a 3-mm camera integrated within a small-bore
feeding tube to allow clinicians to identify anatomical markers
during the placement of a tube.
31
Although observing for respiratory symptoms is warranted
during EAD insertion, malpositioning may occur without any
apparent symptoms.
32,33
The appearance and pH of aspirates
from a feeding tube may provide clues to an EAD location but
has not been shown to be reliable as a single marker for tube tip
location. Fluid withdrawn from a tube that has perforated into
the pleural space typically has a pale yellow serous appearance
and a pH of 7 or higher, whereas fasting gastric fluid typically
is clear and colorless or grassy green or brown with a pH of 5
or less.
34–38
Several studies demonstrating the use of pH testing
indicate a pH of 5.5 from tube aspirate is adequate to check
the position of the tube in the stomach.
The auscultatory method of tube tip confirmation is unreli-
able.
17,39
Multiple case reports clearly indicate that clinicians
cannot differentiate between respiratory and gastric placement
by the auscultatory method.
32,40,41
Several studies have indi-
cated that capnography can be helpful in determining when a
tube has taken the wrong course into the trachea during the
insertion process.
42,43
However, it is important to point out that
this method cannot distinguish between EAD placement in the
esophagus and the stomach. Thus, even though capnography
may indicate nonbronchotracheal placement of a newly
inserted tube, a radiograph is still required to ensure proper
placement in the stomach.
A tube is malpositioned if it is located in the stomach of a
patient receiving small bowel feedings. One study found that
experienced nurses could not distinguish between gastric and

40 Journal of Parenteral and Enteral Nutrition 41(1)
small bowel placement by the auscultatory method.
44
A
higher level of accurate placement has been reported when
clinicians observe the appearance and pH of the feeding tube
aspirate.
45
Small bowel aspirates are typically bile stained,
while fasting gastric fluid is typically clear and colorless or
green or brown.
35
Gastric fluid usually has a lower pH than
that of small bowel secretions. For example, Griffith et al
found that most gastric pH readings were 5, with or without
the use of gastric acid suppression therapy.
37
It should be
noted that when gastric pH is 6, the pH method is of no
benefit in predicting tube location in the GI tract (or in ruling
out tracheopulmonary placement).
After feedings have been started, it is necessary to check
that the tube remains in the desired location (either the stomach
or small bowel). Securing tube with a bridle may be helpful for
preventing accidental dislocation (see below for more detail on
securement). Unfortunately, a small bowel tube may dislocate
upward into the stomach or a gastric tube may migrate down-
ward into the small bowel; a worse scenario is when a tube’s
tip dislocates upward into the esophagus.
46
Obviously, an x-ray
cannot be obtained several times a day to confirm tube loca-
tion; thus, clinicians rely on a variety of bedside methods for
this purpose. Use of the above-mentioned bedside placement
technology (electromagnetic, direct visualization, pH measure-
ment, or CO
2
sensing) can help clinicians to verify tube tip
position. A sharp increase in gastric residual volume may indi-
cate displacement of a small bowel tube into the stomach.
47,48
For long-term EADs, incorrect feeding technique and compli-
cations in tube replacement and removal can result from failure
to recognize the type of tube inserted (gastric vs small bowel),
the insertion technique, and the location of the distal catheter
tip. Follow-up of a long-term percutaneous EAD is indicated to
ensure that the enteral retention device is properly approxi-
mated to the abdominal wall, there is no tube migration, and
excessive tension to the exterior portion of the tube is avoided,
as well as to assess the condition of the surrounding skin.
Question 4.5. What is the best way to confirm accurate
EAD placement and evaluate risk versus benefit of
radiation exposure especially in PEDIATRIC/
NEONATAL patients?
Practice Recommendations
1. Use accurate measurement of enteral tube insertion
length, gastric pH testing, and visual observation of
gastric aspirate as acceptable nonradiologic methods
for assessing tube placement when radiographic
verification is not available.
2. Obtain an abdominal radiograph when other
nonradiographic methods for validation of tube
location are not confirmatory.
3. Avoid using auscultation alone as verification for
nasogastric feeding tube placement.
Rationale
Although placement of a nasogastric tube is a common pro-
cedure, it is not without risk of significant harm or death.
Great care must be taken when placing tubes and confirming
their correct placement. In 2012, the Child Health Patient
Safety Organization issued a safety alert to recommend
immediate discontinuation of the auscultation method for the
assessment and verification of NG tube placement.
49
A study
cited in the alert reported that 1.3%–2.4% of NG tubes in
more than 2000 insertions were located outside the GI tract.
Moreover, more than 20% of the misplaced NG tubes led to
pulmonary complications.
14,49
This alert acknowledges an
abdominal radiograph as the current gold standard when
other nonradiographic methods for validation of tube loca-
tion are not confirmatory.
When abdominal radiography is not readily available or
advisable, the Child Health Patient Safety Organization safety
alert identifies accurate measurement of EAD insertion length,
gastric pH testing, and visual observation of gastric aspirate are
acceptable nonradiologic methods for assessing tube place-
ment listed in the alert.
49
In addition, the alert specifies chil-
dren who are considered at high risk for misplaced or dislodged
gastric enteral tubes: neonates, children with neurological
impairment, children in an obtunded neurological state, and
children who are encephalopathic, have a decreased gag reflex,
or are sedated or critically ill. For these children, the alert rec-
ommends abdominal radiography as the best practice for veri-
fying location of a gastric enteral tube.
49
In addition to the above-mentioned alert, the American
Association of Critical-Care Nurses issued a practice alert
50
and the American Society for Parenteral and Enteral Nutrition
(ASPEN) published practice recommendations
51
to address the
risks and potential complications associated with misplaced
NG tubes.
50,51
Placement of a gastric EAD potentially poses
risks to patient safety, and device dislodgement poses similar
risks.
In a retrospective study of children, Ellett et al
52
demon-
strated by radiographic documentation a prevalence of 21% for
misplaced or dislodged NG, orogastric, and transpyloric tubes.
In a follow-up prospective study, Ellett and Beckstrand
53
used
abdominal radiography to evaluate device placement and
reported a prevalence between 22% and 44% in NG tube place-
ment error in children in their institution, a rate that exceeds the
range found in adult studies. Although alternative methods
exist, abdominal radiographic imaging is the “gold standard”
for verifying NG tube placement.
18,51,54–56
However, even with
radiographs, there may be variation in the interpretation of
device location. This variation is due to a lack of consensus on
identification of specific anatomical landmarks used to verify
the NG tube position within the gastric lumen.
56
In addition,
the lack of a relevant clinical history explaining the need for a
radiograph along with omission of a specific request for device
and device tip location in the radiology requisition can

Boullata et al 41
influence the radiology report.
56,57
Despite these limitations,
radiographic determination is the standard by which all other
methods of verifying NG tube location are measured. An
abdominal or chest radiograph that includes an abdominal
view is considered the most reliable method to document the
course of the tube and its tip location at the time the radiograph
is obtained.
18
Although the radiation exposure associated with a single
abdominal radiograph may be low, repeated exposures for mul-
tiple placement verifications may, over time, result in high
cumulative radiation doses. Both cohort and case-control stud-
ies have associated increased radiation doses with various types
of cancer, including childhood leukemia.
58,59
Moreover, obtain-
ing abdominal radiographs for home care patients and those in
ambulatory and long-term care centers is not practical.
18
The patency and placement of an EAD must be confirmed
before any new EAD is accessed for feeding or medication
administration. Healthcare professionals cannot solely rely on
auscultatory methods to differentiate between gastric and bron-
chopulmonary tube placement because these methods cannot
differentiate between properly placed tubes and tubes improp-
erly placed in the lung or coiled in the esophagus.
8
In pediat-
rics, 2 methods are suggested for tube confirmation. X-ray
confirmation is only valid for that moment in time, as an infant
or child can dislodge the tube quickly. Due to the many times a
pediatric or neonatal tube may be inserted, it may not be rea-
sonable to have x-ray confirmation of each tube placement. In
these situations, when ongoing x-rays are not possible, 2 meth-
ods of tube verification, such as tube length measurement and
pH testing, are recommended.
60
Question 4.6. What are the safe and effective methods to
secure EADs to prevent their displacement?
Practice Recommendations
1. Provide practical education on EAD securement to
clinical staff and assess clinical competencies on a
regular basis.
2. Securement of enterally placed feeding tubes and
prevention of dislodgement are the responsibility of all
clinical staff.
3. Routinely assess patients with EADs to check tube
securement in addition to appropriate tube position.
Early detection of displacement reduces the risk of
adverse events.
4. Consider bridling of nasally placed feeding tubes,
which may help reduce displacement of tubes at risk
for displacement. Understand, however, that there are
insufficient data to recommend this technique on a
routine basis.
5. Include routine assessment in patient monitoring for
signs of tissue pressure, patient discomfort, and
inadequate securement.
a. Pressure on internal tissue related to technique has
not been adequately explored and means to reduce
pressure as well as monitoring for adverse effects
on internal as well as external tissue should be
routine.
6. Use trained staff to periodically assess the appropriate
fit of percutaneous EAD external bolsters and skin
integrity in order to help prevent tissue damage,
leakage, and other issues.
7. Avoid maintaining a bridle for longer than 4 weeks.
Rationale
Nasal tubes. Once the nasally inserted tube has been safely
placed and tip location verified, the challenge is to keep the
tube in place. Nasal feeding tubes are frequently dislodged in
hospitalized patients. In a study of 49 intensive care units by
Mion et al,
61
22.1 episodes of tube dislodgement occurred per
1000 patient days, for a rate of 28.9% for nasogastric tubes.
Not surprisingly, EAD removal has been associated in several
studies with patient agitation, disorientation, and restlessness;
nosocomial infection; and a score of 9 or less on the Glasgow
Coma Scale, as well as medication use.
61,62
Aspiration is among the risks associated with dislodgment
of nasal feeding tubes. The potential for aspiration may be
greatest in patients whose tubes become only partially dis-
placed so that feeding is infused into the pharynx or upper
esophagus, especially if the displacement remains undetected
for a period of time.
Nasal tubes are sometimes taped to the nose, with a type of
wrap around the tube using adhesive tape, partially split ban-
dage, or other material, which then may be pinned to a patient
gown or clothing. The tube typically hangs from the nose,
where it could be a patient distraction, and gown misplace-
ment or change can tug on the tube secured to it. When a nasal
tube is taped to the nose, the taping must be done in a manner
to prevent pressure against surrounding tissue as pressure
sores may develop. Monitoring for pressure on related tissue
must be routine.
Another method of securing the nasal feeding tube uses a
semipermeable transparent dressing from the tube exit at the
naris and across the cheek, as is often noted in pictures of tube
securement for children. Taping the tube to the neck (pinching it
around the tube) provides additional securement in a stable area.
This method may work well for smaller flexible tubes, although
resecurement may be needed as facial hair grows. Skin cleansing
and an adherent agent such as tincture of benzoin may be helpful
in securing the dressing to oily skin. This method is more out of
sight and discreet for the patient than a tube secured to the nose.
If the patient has a visual deficit in one eye, placement of the
tube in the naris and securement on the affected side of the face
may reduce the patient’s temptation to pull at the tube.
Manufactured fixation devices are also sometimes used for
nasal tube securement. These devices may include adhesive

42 Journal of Parenteral and Enteral Nutrition 41(1)
strips or a clip for attachment of the feeding tube, which has
been shown to reduce nasal pressure ulcers. In a study of 205
patients, Ambutas et al
63
reported use of a commercial NG tube
holder was associated with fewer nasal pressure ulcers than use
of typical adhesive tape to secure 14 and 16 French sump
tubes. The results for in this study did not reach statistical sig-
nificance, but the findings were deemed to be clinically mean-
ingful, suggesting that the method of securement may impact
pressure on surrounding tissue.
Nasal tubes may be sutured to the naris in some situations to
reduce inadvertent displacement such as after head and neck
surgery; however, tubes can become displaced partially or com-
pletely through the sutures. The suture disrupts skin integrity,
which carries a risk of infection. Additionally, the patient may
experience discomfort at the suture site, especially if there is
tension on the tube, such as from being snagged or tugged on.
The nasal tube retention device, also known as a “bridle” or
“bridle loop” provides additional securement for patients at
high risk for nasal feeding and occasionally nasogastric suction
tube displacement. Various techniques and materials for bri-
dling tubes have been described. In general, small-bore tubing
or umbilical or twill-type tape wraps around the nasal septum
with each end exiting a naris and the feeding tube is secured to
the bridle tubing or twill tape. It is recommended that staff who
bridle tubes be trained in the technique, demonstrate compe-
tency, and maintain skill through frequency of use.
One method of bridling a feeding tube involves the use of a
manufactured device. This method uses a magnetic retrieval
system to facilitate bridle placement. Another method is con-
structed with materials and supplies available in many nursing
areas. This method involves retrieval of 5 French feeding tubes
that have been inserted through each naris from the oral cavity
and pulling one aspect back through a naris to create a loop
around the posterior aspect of the nasopharynx, which is then
secured to the feeding tube with skin securement strips and
secured to the side of the face.
64
Bridling is associated with low rates of morbidity and com-
plications, the most common being failure to prevent feeding
tube displacement. Even with the bridle in place, patients with
fine motor skills can catch a small loop of the feeding tube
between the naris and bridle and dislodge the tube. Patients
with gross motor skills might tug on the bridled tube, espe-
cially if they do not associate the discomfort they experience
with the tugging. When the small-bore feeding tube is used as
a bridle, it may break and release if it is tugged on firmly,
which may be viewed as a benefit in reducing or preventing
tissue trauma. It is advisable to evaluate the patient for internal
nasoseptal damage if any bridled tube is tugged on signifi-
cantly. Nasal ulceration is another potential complication if
securement is too tight. Ideally, the bridle material does not
cause pressure on internal and/or external tissue or make the
patient uncomfortable by being secured too tightly; however,
the bridle must be secure enough to prevent tube dislodgement.
To prevent undue pressure on the septum from the bridle loop,
the feeding tube might be secured to the patient’s face. Other
potential complications of bridling include sinusitis, bleeding,
patient discomfort, and septal erosion or trauma. In a study of
80 patients randomly assigned to nasal bridle or adhesive
device, bridled tubes were less likely to be dislodged than
unbridled tubes; however, 5 cases of mild epistaxis and 4 cases
of superficial nasal ulceration were associated with the bridle.
65
Two patients presented with retained system insertion stylets
as nasal foreign bodies.
66
It has been suggested that the use of
nasal bridles for greater than 4–8 weeks can result in nasal ero-
sion, although longer term use of the bridle has been reported
with no adverse effects.
67
In a study of burn patients, Parks and colleagues
68
reported
that 17 patients with bridled tubes had significantly fewer tube
insertions than the prebridle control group of 33 patients with
taped tubes. The investigators concluded that the use of a nasal
bridle to secure tubes in burn patients had clinical advantages
over traditional adhesive tape securement. A systematic review
by Brugnolli et al
69
of published and unpublished reports of
nasogastric tube securement in any language found 5 studies, 2
of which were randomized controlled trials. Four studies in
that review compared bridled tubes with unbridled (taped)
tubes and found a favorable advantage for bridled tubes. Three
studies in that review measured time until failure, with 2 com-
paring bridle vs tape methods and the other study comparing
types of tape. Of those 2 studies, one did not find a significant
difference between groups and one demonstrated a longer time
until securement failure in bridled tubes. Three studies com-
paring adverse events in bridled vs nonbridled tubes had con-
tradictory findings. The authors of these studies concluded that
despite the large number of patients receiving this intervention,
“there is insufficient evidence to suggest one securing tech-
nique over another” and “there is little or no statistically sig-
nificant evidence regarding bridling of nasogastric tubes but
more research is needed.” Patient discomfort was not measured
in the studies in this report.
69
A meta-analysis by Bechtold and
colleagues,
67
reviewing patients with nasal bridles, showed
similar results, finding limited data regarding secondary out-
comes such as pain, nasal septal erosion, and epistaxis. Proper
care and technique are suggested to avoid skin irritation, break-
down, and ulceration, and it is important to limit pressure and
remove the bridle once removal is clinically indicated. Bridling
for those at high risk for tube dislodgement may be an effective
strategy for access securement. Consideration of the benefits
of placement of a nasal bridle in the severely agitated patient
must be weighed against the potential for internal septal
trauma. As with any healthcare decision, the clinician and
patient/family must consider the safety, potential benefits, and
potential risks to bridling a nasally placed tube.
Contraindications to the use of the bridle include nasal
trauma or malformation, mechanical obstruction, craniofacial
or basilar skull fractures, and propensity for epistaxis by his-
tory or related to coagulation status. Removal of the bridled
tube is done by cutting one (and only one) aspect of the bridle.

Boullata et al 43
The bridle can then be pulled through the naris along with the
nasally placed tube. This information should be sent with a
patient who has a bridled feeding tube when he or she transfers
to another care facility.
Inability to maintain the nasoenteral route for feedings due
to dislodgement may precipitate a decision point in therapy to
answer the following questions. Will this patient continue on
enteral feeds? Is oral nutrition now possible and will oral intake
adequacy be obtained if EN is stopped? Or, have the goals of
therapy changed? If ongoing EN is still indicated, the place-
ment of a percutaneous EAD (ie, gastrostomy, jejunostomy,
gastrojejunostomy tube) may be necessary to maintain enteral
access. A percutaneous tube placement may be preferred in
patients who are expected to need EN for more than 4–6 weeks
and a more reliable feeding delivery system with a reduction of
tube dysfunctions. However, patients who have repeatedly dis-
lodged nasally placed tubes may also be at risk for dislodging
a percutaneously placed tube, which can have dire clinical con-
sequences, especially soon after placement and prior to tract
maturation. Therefore, a strategy to avoid tube displacement
must be included in the patient plan of care.
Percutaneous tubes. General categories of percutaneous
EAD include gastrostomy, jejunostomy, and gastrojejunos-
tomy tubes. Securement of these tubes is necessary to not
only prevent dislodgment but also to prevent internal migra-
tion related to peristalsis that can result in feeding intolerance
and in gastrostomy tubes; blockage can occur at the pylorus
by the internal fixation device (eg, balloon, rubber bumper or
pigtail loop). Percutaneous EADs typically have external bol-
sters (known as “bumpers” or “disks”) to prevent this inward
migration and are recommended for use as opposed to cathe-
ters not designed for enteral feeding, which may not have
external bolsters (such as urinary catheters). External bolsters
must fit appropriately to prevent both internal and external
pressure (such as buried bumper). Fit should allow for easy
rotation of the tube (gastrostomy tube only; jejunostomy and
gastrojejunal tubes should not be rotated) and permit cleaning
under the bolster (suturing of the bolster to the skin may pro-
hibit cleaning and contribute to irritation; other means to
reduce displacement may be preferable). A slim layer of light
breathable gauze can be inserted under the disc, if indicated.
An external disc that is too loose, permitting internal and
external movement of the tube (positioning), may let gastric
contents leak through the gastrostomy opening, which then
may lead to skin excoriation and other complications. Appro-
priate fit of gastric (and jejunal) tubes and the integrity of
surrounding tissue are key to successful tube usage as well as
patient comfort.
Gastrostomy tubes placed endoscopically typically have an
internal bolster as opposed to a balloon to keep them from
being pulled out. Some types of these tubes may be more chal-
lenging to remove, but other tubes are called “traction remov-
able,” meaning that clinicians can intentionally remove them
with moderate traction for replacement or discontinuation.
However, they can also be inadvertently removed by patients
as well. As a result, means of securement are as important for
these tubes as they are for other tubes. In a study of PEG tubes
placed by one surgeon during a 3-year period, Rosenberger and
colleagues
70
reported a 30-day mortality rate of 7.8%, a 7-day
early dislodgement rate of 4.1%, and a lifetime early accidental
dislodgement rate of 12.8% (72 of the 563 PEG tubes).
Internal balloons can secure gastrostomy or gastrojejunos-
tomy tubes that are placed using fluoroscopy or open or lapa-
roscopic surgical procedures. Tubes can potentially be pulled
out with the balloon intact; additionally, balloons may rupture
or slowly loose ability to hold fluid, resulting in tube loose-
ness and dislodgement. Michaud et al
71
evaluated 165 gastros-
tomy tubes for 84 children and reported that the mean
longevity of the balloon-type low-profile gastrostomy tube
was 5 months (range, 14 days to 14 months). In that study,
balloon failure was not correlated with underlying disease,
age of the patient, or the use of antisecretory drugs. Some
companies highlight the strength of the balloon, including
low-profile tubes. However, literature on outcomes of specific
internal fixation methods is sparse, and clinicians should fol-
low manufacturer’s guidelines for frequency of tube changes.
Another internal fixation method uses a pigtail-type catheter
where the internal end becomes looped when pulled on by an
external string. One brand of gastrostomy tube has a right
angle feature, which results in a lower profile and may have
the advantage of being more discreet.
Low-profile, skin-level, or button-type tubes are used fre-
quently, especially in children, although many adults also
appreciate these types of EAD. They are less bulky to pull on
(especially when not accessed by an adaptor for feeding), are
less visible under clothing, contain antireflux valves, and do
not require tape to secure them to the abdomen. However, they
may cost more than other standard gastrostomy tubes. Use of a
low-profile device requires periodic resizing in growing chil-
dren to prevent compression injuries of the gastric mucosa or
epidermis.
Accidental removal of gastrostomy tubes within 2–4 weeks
of placement before the tract has matured may result in perito-
nitis and even death if gastric content leaks into the perito-
neum.
72–74
Excessive traction on the tube in this period may also
cause peritonitis if the bolster gets pulled through the gastric/
jejunal wall.
75
Techniques such as gastropexy using temporary
sutures or T-fasteners to secure the stomach to the abdominal
wall until it affixes to the abdominal wall can help reduce
potential for leakage into the peritoneum and aid in easier and
safer tube replacement when needed.
76–79
These devices must
be monitored to be sure they are not causing pressure on the
skin.
Jejunostomy tubes might have low-volume (eg, 3–4 mL)
balloons to prevent tube displacement. However, balloons
can be dislodged in the tract. Fit of the bolster and care of
surrounding tissue are paramount to successful use of these

44 Journal of Parenteral and Enteral Nutrition 41(1)
tubes. To reduce pressure and tugging on the tube, it is impor-
tant to adequately secure the tube to the abdomen with an
appropriate tape (fixing tape around the tube, then to the skin)
or another method of securement. Some jejunal tubes have a
Dacron cuff that becomes embedded in the subcutaneous tis-
sue and can help prevent displacement and serve as success-
ful jejunal access for years. Secure these tubes low to the skin
to allow the cuff to embed (as opposed to padding under the
tube itself) and also to the abdomen, at least until the cuff is
well embedded.
Other tubes (eg, red rubber catheters) may not have an
internal means of fixation. If these tubes are not well secured,
external migration resulting in dislodgement can occur more
easily than with other types of tubes. In addition, if this type
of tube irritates and reddens patient skin, securing the tube to
the irritated skin may be difficult. External fixation devices
are not always necessary or fail-proof. If they are used, the
skin under them must be carefully monitored for moisture
retention, which can lead to microbial growth and tissue
breakdown.
Strategies to prevent tube dislodgement. Patients can cause
serious harm to themselves by removing tubes essential for
breathing or feeding. In the past, the use of medications or
physical restraints, including wrist restraints or mittens, was
suggested for high-risk patients. However, the use of physical
restraints may actually increase a state of delirium and/or agi-
tation and in turn contribute to tube removal by the patient.
62
The use of physical or chemical restraints is rightfully dis-
couraged in today’s healthcare environment. Alternative
methods of securing tubes are advisable whenever and wher-
ever their use is possible. Healthcare providers can also reduce
risk of dislodgement by talking to patients and orienting them
in a calm, person-centered manner, as people are generally
more cooperative when they are well informed.
Healthcare organizations may choose to employ staff to
prevent self-harm by patients without use of restraints. This
approach may be effective, but it is an added expense. Family
members who are visiting or caring for the patient are some-
times asked to monitor for patient safety. The range of effective
alternatives to restraints may expand as patient cognition
improves.
The acronym MARK can be used to guide steps for moni-
toring tube securement. M is for marking the tube at the exit
with an indelible marker to help identify displacement at a
glance. It is also important to record the external length at the
time of placement by using the number on the tube at the exit
site, which is often denoted in centimeters; this number can be
used to monitor for feeding tube migration. A is for anchoring
the tube (as previously discussed). R is for reassessment of the
tube placement. Frequent reassessment is advisable, especially
in patients at risk for displacement as well as during procedures
that increase risk of dislodgement, such as patient positioning
and transfers. K has 2 meanings. It stands for keeping pressure
off of skin or the septum, and it refers to the knowledge needed
to ensure safe practice in policy, procedure, and clinical prac-
tice (Lorraine Linford, personal communication).
Other recommendations for preventing displacement of
long-term tubes include using an abdominal binder for those
at risk for pulling at tubes, using a gastrostomy tube that has
an internal bolster that “cannot” be removed with traction
(requires endoscopic removal), and changing to a low-profile
tube.
80
Tubes can be hidden inside of a tucked-in shirt and
other creative strategies can be used to secure the tube and
keep it “out of sight and out of mind,” reducing patient focus
on the tube. If a tube securement device is used, the potential
exists for moisture to be trapped under the coverage area until
the device is replaced, which may be days, due to cost or pro-
tocol. Moisture retention can promote microbial growth and
potential skin breakdown. Therefore, careful assessment and
monitoring are recommended when tube securement devices
are used. Feedings can be scheduled so that the patient receives
needed feeding over shorter periods, such as gravity bolus
feeding for gastric tubes, when more staff are available for
monitoring, or jejunal delivery of feedings can be scheduled at
night or day to reduce periods when the tube is accessed.
Additionally, follow-up by trained personnel is key to pre-
venting tube displacement, other issues (such as buried bum-
per due to tightness of securement), and minimizing problems
if a tube should become displaced.
75,81
Key strategies to reduce or prevent tube displacement
include education of staff at inpatient and transfer facilities,
education of patients and their families, and monitoring of prac-
tice and performance improvement projects. To ensure safety
and efficacy while maintaining dignity and comfort for the
patient as possible, the clinician is advised to use researched as
well as innovative noninvasive methods to secure enteral tubes.
Question 4.7. How soon after placement of a long-term
percutaneous endoscopic gastrostomy (PEG) can
feedings begin?
Practice Recommendations
1. Use a PEG tube for feedings within several hours of
placement. Current literature supports 4 hours in
adults and children.
2. Educate providers on the appropriate timing of use of
the PEG tube postprocedure.
3. Review procedural documentation for time of PEG
insertion.
Rationale
Traditionally, tube feedings have been delayed after percuta-
neous placement of gastrostomy tubes to the next day and up
to 24 hours after the procedure. No consensus exists regarding
feeding initiation after placement. In 2011, a national survey

Boullata et al 45
of practicing gastroenterologists found variation in the timing
of feeding.
82
The response rate for the questionnaire was 28%
(n = 1474), and 41% of the respondents were aware of the cur-
rent literature on post-PEG feeding times. Those aware of the
current literature were more likely to initiate early feeding.
Eight percent of the respondents initiated feedings in general
ward patients within the first 3 hours, and 32.5% initiated
feedings 4–6 hours post-PEG in the same patient population.
82
Bechtold and colleagues
83
pooled the results of 6 randomized
controlled trials that compared early (range, 1–4 hours) vs
delayed feeding after PEG placement and found no statisti-
cally significant differences in complications or death in the
first 72 hours after PEG placement.
A meta-analysis of 5 randomized controlled trials compared
early feeding after PEG placement (3 hours) with delayed or
next-day feeding and found no significant differences in com-
plications, deaths in the first 72 hours, or number of significant
gastric residual volumes at day 1.
84
A retrospective study
examined the safety and outcomes of early feedings (4 hours)
during a 5-year period at a tertiary care center where the major-
ity of PEG procedures were performed on inpatients by gastro-
enterologists.
85
The mean time of feeding was 3.2 hours for the
early group (4 hours) vs the delayed (>4 hours) feedings for
either overall mortality within 30 days or overall complications
such as wound infection, melena, vomiting, leakage, stomati-
tis, and aspiration pneumonia.
85
Kim and associates
86
evalu-
ated the feasibility and safety of implementation of an early
tube use protocol compared with the institution’s policy on
4-hour post-PEG tube placement checks. The early tube use
protocol involves immediate tube assessment by the gastroen-
terology fellow after return of the patient’s preprocedure level
of consciousness (within 1 hour of procedure completion).
This study showed that an immediate-use protocol with a
prompt assessment following recovery from sedation seems
safe and effective. The difference in the rates of complication
between the 4-hour placement group and the immediate-use
group was not significant.
86
In pediatric patients, the earliest reported time of feeding ini-
tiation after PEG tube placement had been 6 hours. Corkins
et al
87
randomly assigned pediatric patients to use a PEG tube for
feedings at 3 hours and 6 hours after placement. The researchers
documented the change in abdominal girth from before the ini-
tial feeding to 1 hour after, any vomiting, and the gastric residual
volume before the next feeding. The initial feeding was limited
to Pedialyte (Abbott Nutrition, Columbus, OH) at a volume of
60 mL bolus feeding. The authors concluded that feedings after
PEG tube placement can be started as soon as 3 hours with no
increase in complications.
87
A recent prospective randomized
controlled study compared early (4th hour) and late (12th hour)
feeding after a PEG procedure in 69 children.
88
This study
showed that initiation of feedings at the 4th hour was safe and
well tolerated by patients and shortened the duration of the hos-
pital stay.
88
In a retrospective chart review of 70 pediatric
patients, the early initiation of feedings (6 hours post-PEG) also
led to a shortened length of hospital stay with no increase in
adverse events or reported pain.
89
Question 4.8. How often should you replace long-term
EADs?
Practice Recommendations
1. Develop institutional protocols for replacing
percutaneous EADs that reflect manufacturers’
guidelines:
a. Routine removal and replacement of a well-
maintained percutaneous EAD may not be
necessary.
b. Replace per manufacturer guidelines.
2. Consider tube replacement sooner than indicated in
manufacturer guidelines if any of the following are
identified:
a. Deterioration and dysfunction of the EAD
b. A ruptured internal balloon
c. Stomal tract disruption
d. Peristomal infection that persists despite
appropriate antimicrobial treatment
e. Skin excoriation
f. Nonhealing ulcer formation that will not heal
despite good wound care technique
g. Colocutaneous fistula or gastrocolic fistulas
3. Replace the percutaneous tube only after the stoma
tract has fully matured (30–90 days from initial
insertion) or per institutional protocols.
4. Consider routine replacement of the percutaneous tube
after the stoma tract has matured (>30 days from initial
insertion) or per institutional protocols.
Rationale
Eventually, tubes will require replacement. The most common
indications for replacement include tube deterioration over
time, inadvertent removal, device-related complications (leak-
ing, unrestorable tube patency), fistula disruption, peristomal
infection, skin excoriation, ulcer formation, colocutaneous or
gastrocolic fistulas, or the device is being changed to a low-
profile gastrostomy tube.
8,90,91
In patients with a PEG tube, most major complications have
been reported to occur within the first few days of initial tube
placement when the tube tract is not yet mature. The tract
begins to mature approximately 7–10 days after PEG place-
ment, and it takes a few weeks for fusion to take place between
the stomach and peritoneum. In malnourished or immunocom-
promised patients, this process can take longer.
84
Patients who
are discharged home with a newly inserted PEG tube must be
closely monitored to prevent inadvertent dislodgement. If the
gastrostomy tube dislodges in the first 7–10 days after inser-
tion, the inserting provider needs to be contacted as soon as

46 Journal of Parenteral and Enteral Nutrition 41(1)
possible for further intervention. A dislodged PEG tube can
become a medical emergency, as stomach contents are likely to
leak into the peritoneum. The tube should not be reinserted
blindly at this stage because it may be repositioned into the
peritoneum. Possible approaches to management include
immediate reinsertion under radiographic or endoscopic guid-
ance, laparotomy, or conservative management (cessation of
oral intake, nasogastric suction, and antibiotics) followed by
reinsertion in 7–10 days.
21,80
If displacement occurs after the
tract is mature (>30 days), prompt replacement with a percuta-
neously balloon gastrostomy tube is recommended.
92
DiBaise and associates
93
evaluated the rate of tract disrup-
tion in adults requiring long-term EN and found that tract dis-
ruption occurs infrequently during replacement of gastrostomy
tubes. Tract disruption seems to be an issue primarily during
the initial tube exchange using a skin-level device. The mini-
mum duration to wait before there is sufficient tract maturity to
allow for safe gastrostomy replacement remains unknown, and
the authors recommend waiting at least 2 or 3 months for safe
tube replacement.
86
Most gastrostomy tubes with internal bolsters (ie, PEG
tubes) use soft, deformable internal bolsters, allowing transcu-
taneous replacement to be performed without endoscopy. A
credentialled provider can remove the device from the skin by
traction. In this procedure, the bolster is extended linearly so
that it passes through the gastrocutaneous stomal tract. A new
percutaneous EAD is then inserted through the gastrocutane-
ous stoma. The new EAD may be either a balloon gastrostomy
tube that is followed by balloon inflation or a nonballoon bol-
ster that is stretched taut by using an obturator and then
released. With optimal care, most bolster-type gastrostomy
tubes may remain in place for up to 1–2 years.
8
Manufacturers
often do not recommend such a long duration because internal
bolsters can wear off and potentially obstruct the GI tract. In
accordance with the manufacturers’ recommendations, some
institutions routinely replace EADs at 6-month intervals,
before the deformability of the bolster decreases.
87
Preventive
maintenance of balloon gastrostomy tubes, which includes
elective change at a fixed time interval (such as every 3–6
months), is the standard of practice in some facilities because
of the potential for balloon failure.
8
Topics for Future Research
Comparison of nonradiographic methods of confirming
tube position to abdominal x-ray
Communication about EN during the transition of care
and confirmation of EAD placement and EN orders
after transition of care
Obstacles and/or barriers in standardizing post-PEG
feeding practices
Optimal timing for initiation of feeding for other types
of percutaneous gastrostomy tubes (eg, balloon G-tubes,
gastrojejunostomy tubes, and jejunostomy tubes)
The frequency of malposition and peritonitis after PEG
and balloon gastrostomy tube replacement
The optimal protocol for PEG and balloon tube
replacement
References
1. Löser C, Aschl G, Hébuterne X, et al. ESPEN guidelines on artificial
enteral nutrition—percutaneous endoscopic (PEG)—consensus statement.
Clin Nutr. 2005;24:848-861.
2. Norton B, Homer-Ward M, Donnelly MT, et al. A randomized prospective
comparison of percutaneous endoscopic gastrostomy and nasogastric tube
feeding after acute dysphagic stroke. BMJ. 1996;312:13-16.
3. Park RHR, Allison MC, Lang J, et al. Randomized comparison of
percutaneous endoscopic gastrostomy and nasogastric tube feeding
in patients with persisting neurological dysphagia. BMJ. 1992;304:
1406-1409.
4. Vermilyea S, Goh VL. Enteral feedings in children: sorting out tubes, but-
ton and formulas. Nutr Clin Pract. 2016;31(1):59-67.
5. Axelrod D, Kazmerski K, Iyer K. Pediatric enteral nutrition. JPEN J
Parenter Enteral Nutr. 2006;30(1)(suppl):S21-S26.
6. Joffe A, Anton N, Lequier L, et al. Nutritional support for critically ill
children. Cochrane Database Syst Rev. 2009;(2):CD005144.
7. Day AS, Burgess L. Exclusive enteral nutrition and induction of remis-
sion of active Crohn’s disease in children. Expert Rev Clin Immunol.
2013;9(4):375-384.
8. Itkin M, DeLegge MH, Fang JC, et al. Multidisciplinary practical
guidelines for gastrointestinal access for enteral nutrition and decom-
pression from the Society of Interventional Radiology and American
Gastroenterological Association (AGA) Institute, with Endorsement
by Canadian Interventional Radiological Association (CIRA) and
Cardiovascular and Interventional Radiological Society of Europe
(CIRSE). Gastroenterology. 2011;141:742-765.
9. Bjuresater K, Larsson M, Nordström G, Athlin E. Cooperation in the care
for patients with home enteral tube feeding throughout the care trajectory:
nurses’ perspectives. J Clin Nurs. 2008;17:3021-3029.
10. Silver HJ, Wellman NS, Arnold DJ, et al. Older adults receiving home
enteral nutrition: enteral regimen, provider involvement, and health care
outcomes. JPEN J Parenter Enteral Nutr. 2004;28(2):92-98.
11. Hesselink G, Zegers M, Vernooij-Dassen M, et al. Improving patient dis-
charge and reducing hospital readmissions by using intervention mapping.
BMC Health Serv Res. 2014;14:389.
12. Agency for Clinical Innovation and the Gastroenterological Nurses
College of Australia. A Clinician’s Guide: Caring for People With
Gastrostomy Tubes and Devices. Chatswood, Australia: Agency for
Clinical Innovation; 2014.
13. Krenitsky J. Blind placement of feeding tubes: treatment of threat? Pract
Gastroenterol. 2011;35:32-42.
14. Sorokin R, Gottlieb JE. Enhancing patient safety during feeding-tube
insertion: a review of more than 2000 insertions. JPEN J Parenter Enteral
Nutr. 2006;30(5):440-445.
15. Marderstein E, Simmons R, Ochos J. Patient safety: effect of institutional
protocols on adverse events related to feeding tube placement in the criti-
cally ill. J Am Coll Surg. 2004;199(1):39-50.
16. Koopman MC. A team-based protocol and electromagnetic technology
eliminate feeding tube complications. Ann Surg. 2011;253:297-302.
17. Turgay AS, Khorshid L. Effectiveness of the auscultatory and pH meth-
ods in predicting feeding tube placement. J Clin Nurs. 2010;19(11-12):
1553-1559.
18. Farrington M, Lang S, Cullen L, Stewart S. Nasogastric tube place-
ment verification in pediatric and neonatal patients. Pediatr Nurs.
2009;35(1):17-24.
19. Ellett MLC. What is known about methods of correctly placing gastric
tubes in adults and children. Gastroenterol Nurs. 2004;27(6):253-259.

Boullata et al 47
20. Sparks DA, Chase DM, Coughlin LM, Perry E. Pulmonary complications
of 9931 narrow-bore nasoenteric tubes during blind placement: a critical
review. JPEN J Parenter Enteral Nutr. 2011;35(5):625-629.
21. Baskin WN. Acute complications associated with bedside placement of
feeding tubes. Nutr Clin Pract. 2006;21:40-55.
22. Metheny NA, Meert KL. Monitoring feeding tube placement. Nutr Clin
Pract. 2004;19:487-495.
23. Metheny NA, Meert KL, Clouse RE. Complications related to feeding
tube placement. Curr Opin Gastroenterol. 2007;23:178-182.
24. Miller KR, McClave SA, Kiraly LN, Martindale RG, Benns MV. Tutorial
on enteral access in adult patients in the hospitalized setting. JPEN J
Parenter Enteral Nutr. 2014;38(3):282-295.
25. Munera-Seeley V, Ochoa JB, Brown N, et al. Use of a colorimetric car-
bon dioxide sensor for nasoenteric feeding tube placement in critical
care patients compared with clinical methods and radiography. Nutr Clin
Pract. 2008;23(3):318-321.
26. Powers J, Luebbehusen M, Spitzer T, et al. Verification of an electro-
magnetic placement device compared with abdominal radiograph to pre-
dict accuracy of feeding tube placement. JPEN J Parenter Enteral Nutr.
2011;35(4):535-539.
27. Smithard D, Barrett NA, Hargroves D, Elliot S. Electromagnetic sen-
sor-guided enteral access systems: a literature review. Dysphagia.
2015;30(3):275-285.
28. Bear DE, Champion A, Lei K, et al. Use of an electromagnetic device
compared with chest X-ray to confirm nasogastric feeding tube position in
critical care. JPEN J Parenter Enteral Nutr. 2016; 40(4):581-586.
29. Metheny NA, Meert KL. Effectiveness of an electromagnetic feeding tube
placement device in detecting inadvertent respiratory placement. Am J
Crit Care. 2014;23:240-248.
30. Bryant V, Phang J, Abrams K. Verifying placement of small-bore feed-
ing tubes: device images versus abdominal radiographs. AJCC Am J Crit
Care. 2015;24(6):525-530.
31. Coronado M, Engracia E, Fabella A, et al. Electromagnetic guidance feed-
ing tube placement: elimination of kub verification. Clinical Nutrition
Week Abstract M-29 January 2016. http://pen.sagepub.com/content/
suppl/2015/12/17/40.1.115.DC3/CNW16_Monday_Poster_Abstracts_
revised.pdf. Accessed August 7, 2016.
32. Rassias A, Ball P, Corwin HL. A prospective study of tracheopulmonary
complications associated with the placement of narrow-bore enteral feed-
ing tubes. Crit Care. 1998;2:25-28.
33. Metheny N, Dettenmeier P, Hampton K, Wiersema L, Williams P.
Detection of inadvertent respiratory placement of small-bore feeding
tubes: a report of 10 cases. Heart Lung. 1990;19:631-638.
34. Metheny NA, Clouse RE, Clark JM, Reed L, Wehrle MA, Wiersema L.
Techniques & procedures: pH testing of feeding-tube aspirates to deter-
mine placement. Nutr Clin Pract. 1994;9:185-190.
35. Metheny N, Reed L, Berglund B, Wehrle MA. Visual characteristics of
aspirates from feeding tubes as a method for predicting tube location. Nurs
Res. 1994;43:282-287.
36. Metheny NA, Reed L, Wiersema L, McSweeney M, Wehrle MA, Clark J.
Effectiveness of pH measurements in predicting feeding tube placement:
an update. Nurs Res. 1993;42:324-331.
37. Griffith DP, McNally AT, Battey CH, et al. Intravenous erythromycin
facilitates bedside placement of postpyloric feeding tubes in critically ill
adults: a double-blind, randomized, placebo-controlled study. Crit Care
Med. 2003;31:39-44.
38. Metheny NA, Aud MA, Ignatavicius DD. Detection of improperly posi-
tioned feeding tubes. J Healthc Risk Manag. 1998;18:37-48.
39. Boeykens K, Steeman E, Duysburgh I. Reliability of pH measurement and
the auscultatory method to confirm the position of a nasogastric tube. Int J
Nurs Stud. 2014;51(11):1427-1433.
40. Harris CR, Filandrinos D. Accidental administration of activated charcoal
into the lung: aspiration by proxy. Ann Emerg Med. 1993;22:1470-1473.
41. Torrington KG, Bowman MA. Fatal hydrothorax and empyema compli-
cating a malpositioned nasogastric tube. Chest.1981;79:240-242.
42. Asai T. Use of a capnograph during feeding tube insertion. Crit Care Med.
2002;30:1674.
43. Burns SM, Carpenter R, Truwit JD. Report on the development of a pro-
cedure to prevent placement of feeding tubes into the lungs using end-tidal
CO
2
measurements. Crit Care Med. 2001;29:936-939.
44. Metheny N, McSweeney M, Wehrle MA, Wiersema L. Effectiveness of
the auscultatory method in predicting feeding tube location. Nurs Res.
1990;39:262-267.
45. Gharpure V, Meert KL, Sarnaik AP, Metheny NA. Indicators of postpy-
loric feeding tube placement in children. Crit Care Med. 2000;28:2962-
2966.
46. Metheny NA, Spies M, Eisenberg P. Frequency of nasoenteral tube dis-
placement and associated risk factors. Res Nurs Health. 1986;9:241-247.
47. Metheny NA, Schnelker R, McGinnis J, et al. Indicators of tubesite during
feedings. J Neurosc Nurs. 2005;37:320-325.
48. Welch SK, Hanlon MD, Waits M, Foulks CJ. Comparison of four bedside
indicators used to predict duodenal feeding tube placement with radiogra-
phy. JPEN J Parenter Enteral Nutr. 1994;18:525-530.
49. National Association of Children’s Hospitals (NACH), ECRI Institute.
Blind Pediatric NG Tube Placements Continue to Cause Harm. Overland
Park, KS: Child Health Patient Safety Organization; 2012.
50. American Association of Critical-Care Nurses (AACN). AACN Practice
Alert: initial and ongoing verification of feeding tube placement in adults.
CriticalCareNurse. 2016;36(2):e8-e13.
51. Bankhead R, Boullata J, Brantley S, et al. A.S.P.E.N enteral nutrition prac-
tice recommendations. JPEN J Parenter Enteral Nutr. 2009;33(2):122-
167.
52. Ellett MLC, Maahs J, Forsee S. Prevalence of feeding tube placement
errors and associated risk factors in children. MCN Am J Maternal Child
Nurs. 1998;23(5):234-239.
53. Ellett MLC, Beckstrand J. Examination of gavage tube placement in chil-
dren. J Soc Pediatr Nurs. 1999;4(2):51-60.
54. Longo MA, Society of Pediatric Nurses (SPN) Clinical Practice
Committee, SPN Research Committee. Best evidence: nasogastric tube
placement verification. J Pediatr Nurs. 2011;26(4):373-376.
55. Ellett MLC. Important facts about intestinal feeding tube placement.
Gastroenterol Nurs. 2006;29(2):112-123.
56. Cohen MD, Ellett MLC, Perkins SM, Lane KA. Accurate localiza-
tion of the position of the tip of a naso/orogastric tube in children:
where is the location of the gastro-esophageal junction? Pediatr Radiol.
2011;41(10):1266-1271.
57. Cohen MD, Ellett M. Different patterns of reporting the location of the tip
of a nasogastric tube. Acad Radiol. 2012;19(6):651-653.
58. Hart S. Ionizing radiation: promoting safety for patients, visitors and staff.
Nurs Standard. 2006;20(47):47-57.
59. Kleinerman RA. Cancer risks following diagnostic and therapeutic radia-
tion exposure in children. Pediatr Radiol. 2006;36(suppl 2):121-125.
60. Gilbertson HR, Rogers EJ, Ukoumunne OC. Determination of a practical
pH cutoff level for reliable confirmation of nasogastric tube placement.
JPEN J Parenter Enteral Nutr. 2011;35(4):540-544.
61. Mion LC, Minnick AF, Leipzig RM, Catrambone CD, Johnson ME.
Patient-initiated device removal in intensive care units: a national preva-
lence study. Crit Care Med. 2007;35(12):2714-2720.
62. Chang L, Wang KK, Chao Y. Influence of physical restraint on unplanned
extubation of adult intensive care patients: a case-control study. Am J Crit
Care. 2008;17(5):408-415.
63. Ambutas S, Staffileno BA, Fogg L. Reducing nasal pressure ulcers with an
alternative taping device. Medsurg Nurs. 2014;23(2):96-100.
64. McGinnis CM. The feeding tube bridle: one inexpensive, safe, and effec-
tive method to prevent inadvertent feeding tube dislodgement. Nutr Clin
Pract. 2011;26(1):70-77.
65. Seder CW, Stockdale W, Hale L, Janczyk RJ. Nasal bridling decreases
feeding tube dislodgment and may increase caloric intake in the surgi-
cal intensive care unit: a randomized controlled trial. Crit Care Med.
2010;38(3):797-801.

48 Journal of Parenteral and Enteral Nutrition 41(1)
66. Jackson RS, Sharma S. Retained nasal tube bridle system insertion stylet
presenting as nasal foreign body: a report of two cases. Am J Otolaryngol.
2015;36(2):296-298.
67. Bechtold ML, Nguyen DL, Palmer LB, Kiraly LN, Martindale RG,
McClave SA. Nasal bridles for securing nasoenteric tubes: a meta-analy-
sis. Nutr Clin Pract. 2014;29(5):667-671.
68. Parks J, Klaus S, Staggs V, Pena M. Outcomes of nasal bridling to secure
enteral tubes in burn patients. Am J Crit Care. 2013;22(2):136-142.
69. Brugnolli A, Ambrosi E, Canzan F, Saiani L; Naso-Gastric Tube Group.
Securing of naso-gastric tubes in adult patients: a systematic review. Int J
Nurs Stud. 2014;51(6):943-950.
70. Rosenberger LH, Newhook T, Schirmer B, Sawyer RG. Late accidental
dislodgement of a percutaneous endoscopic gastrostomy tube: an under-
estimated burden on patient and the health care system. Surg Endosc.
2011;25(10):3307-3311.
71. Michaud L, Guimber D, Blain-Stregloff AS, Ganga-Zanzou F, Turck D.
Longevity of balloon-stabilized skin-level gastrostomy device. J Pediatr
Gastroenterol Nutr. 2004;38(4):426-429.
72. O’Rear JM, Prahlow JA. Early percutaneous endoscopic gastrostomy tube
dislodgment. Am J Nurs. 2015;115(6):26-31.
73. McClave SA, Chang W. Complications of enteral access. Gastrointest
Endosc. 2003;58(5):739-751.
74. McClave SA, Jafri NS. Spectrum of morbidity related to bolster place-
ment at time of percutaneous endoscopic gastrostomy: buried bumper syn-
drome to leakage and peritonitis. Gastrointest Endoscopy Clin North Am.
2007;17:731-746.
75. Westaby D, Young A, O’toole P, Smith G, Saunders DS. The provision of a
percutaneously placed enteral tube feeding service. Gut. 2010;59:1592-1605.
76. Huan SY, Engstrom BI, Lungren MP, Kim CY. Management of dysfunc-
tional catheters and tubes inserted by interventional radiology. Semin
Intervent Radiol. 2015;32(20):67-77.
77. Lyon SM, Pascoe DM. Percutaneous gastrostomy and gastrojejunostomy.
Semin Intervent Radiol. 2004;21(3):181-189.
78. Dormann AJ, Wejda B, Kahl S, Huchzermeyer H, Ebert MP, Malfertheiner
P. Long-term results with a new introducer method with gastropexy for
percutaneous endoscopic gastrostomy. Am J Gastro. 2006;101:1229-1234.
79. Chadha KS, Thatikonda C, Schiff M, Nava H, Sitrin M. Outcomes of
percutaneous endoscopic gastrostomy tube placement using a T-fastener
gastropexy device in head and neck and esophageal cancer patients. Nutr
Clin Pract. 2010;25(6):658-662.
80. DeLegge M. Percutaneous endoscopic gastrostomy. Am J Gastroenterol.
2007;102:2620-2623.
81. McClave SA, Neff RL. Care and long-term maintenance of percutane-
ous endoscopic gastrostomy tubes. JPEN J Parenter Enteral Nutr.
2006;30S1:S27-S38.
82. Ali T, Le V, Sharma T, et al. Post-PEG feeding time: a web based national
survey amongst gastroenterologists. Digest Liver Dis. 2011;43:768-771.
83. Bechtold ML, Matteson ML, Choudhary A, Puli SR, Jiang PP, Roy PK.
Early versus delayed feeding after placement of a percutaneous endoscopic
gastrostomy: a meta-analysis. Am J Gastroenterol 2008;103(11):2919-
2924.
84. Szary NM, Murtaza A, Matteson ML, Choudhary A, Puli SR, Bechtold
ML. Enteral feeding within three hours after percutaneous endo-
scopic gastrostomy placement: a meta-analysis. J Clin Gastroenterol.
2011;45(4):e34-e38.
85. Cobell WJ, Hinds AM, Nayani R, et al. Feeding after percutaneous endo-
scopic gastrostomy: experience of early versus delayed feeding. South
Med J. 2014;107(5):308-311.
86. Kim AH, Miao CL, Johal AS, Khara HS. Determining the safety and
efficacy of an immediate use strategy following percutaneous endo-
scopic gastrostomy (PEG) tube placement. Gastrointest Endosc.
2015;18(5S):AB231.
87. Corkins MR, Fitzgerald JF, Gupta SK. Feeding after percutaneous endo-
scopic gastrostomy in children: early feeding trial. J Pediatr Gastroenterol
Nutr. 2010;50(6):625-627.
88. Islek A, Sayar E, Yilmaz A, Artan R. Percutaneous endoscopic gastros-
tomy in children: is early feeding safe? J Pediatr Gastroenterol Nutr.
2013;57(5):659-662.
89. Paul F, Perkins J, Jiang H, McCabe M. Impact of the early initiation
of feedings on hospital length of stay in children post-PEG placement.
Gastroenterol Nurs. 2014;37(5):344-349.
90. Nishiwaki S, Araki H, Fang JC et al. Retrospective analyses of com-
plications associated with transcutaneous replacement of percutaneous
gastrostomy and jejunostomy feeding devices. Gastrointest Endosc.
2011;74(4):784-787.
91. American Society for Gastrointestinal Endoscopy. Technology status
evaluation report: Enteral nutrition access devices. Gastrointest Endosc.
2010;72(2):236-248.
92. Taheri MR, Singh H, Duerksen DR. Peritonitis after gastrostomy tube
replacement: a case series and review of literature. JPEN J Parenter
Enteral Nutr. 2011;35(1):56-60.
93. DiBaise JK, Rentz L, Crowell MD, Anton Decker G, Lunsford T.
Tract disruption and external displacement following gastrostomy
tube exchange in adults. JPEN J Parenter Enteral Nutr. 2010;34(4):
426-430.
Section 5. Procure, Select/Prepare, Label,
and Dispense EN
Background
With a wide variety of available EN products on the market,
each organization makes clinical and fiscal decisions to estab-
lish an EN formulary. Each EN product, including human
breast milk (HBM), procured and stocked within a facility,
needs to be uniquely recognized by clinicians involved in EN
therapy. Selection errors can occur when products have similar
names or product labels. Whether dispensed from a central
location or stocked on a patient care unit, EN products must be
labeled to identify the intended patient, date of feeding, and
duration of feeding. Some patients receive EN products that
require preparation from powdered form, which increases the
complexity and safety risk of EN use.
Question 5.1. How is a clinically appropriate and cost-
effective formulary developed, and which experts
should be involved in its development?
Question 5.2. How are EN product shortages and
substitutions managed?
Practice Recommendations
1. Establish a formulary of available EN formulas specific
to the needs of the institution’s patient population.
a. Base the size of the enteral formulary on the
specific needs of the facility, but limit the size to
avoid product duplication, decrease inventory
management, and lower costs.
b. Prioritize formulas that meet the estimated
nutrient needs of patients rather than the patient’s
diagnosis. Use evidence-based research to
evaluate the inclusion of specialty formulas on
the formulary.

Boullata et al 49
c. Consider whether competitive bidding, group
purchasing organizations, or the selection of all
products from the same manufacturer can be cost-
effective. If the facility participates in a corporate
buying group, optimize the contractual agreement
to allow for the purchase of a formula outside of
the formulary if it better meets patients’ nutrition
needs.
2. Develop a multidisciplinary formulary selection
committee of clinicians and administrators, including
dietitians, nurses, pharmacists, and physicians.
3. Generate a substitution list for each EN formula during
the development or restructuring of the EN formulary,
which can be implemented in the case of product
shortages.
4. Allow enough flexibility in the EN process to respond
to manufacturer revisions to their product lines, as well
as product shortages or outages.
Rationale
Over 200 different commercially prepared EN formulas are
available for neonatal, pediatric, and adult use. Beyond stan-
dard formulas, a myriad of specialty formulas are marketed
for specific disorders and disease states. As it is not practical
or cost-effective to provide all available formulas, healthcare
facilities create enteral formularies to control inventory and
cost. In one study published in 1989, more than 75% of the
hospitals had developed EN formularies. The documented
reasons were cost containment, decreased product duplica-
tion, staff education, and inventory management.
1
Another
method to control costs is participation in a group purchasing
organization. Group purchasing may allow healthcare facili-
ties to control costs while providing the best patient care.
Typically, an established commitment level is set for institu-
tional compliance and results in benefits for the purchase of
products and services at lower costs.
2,3
Organizations can
request a clause in the contract to allow for the purchase of a
noncompeting product without penalty if it better meets the
patients’ needs.
The multidisciplinary formulary selection committee will
represent the perspectives of dietitians, nurses, pharmacists,
physicians, and administrators. The committee evaluates the
institution’s patient population and its specific nutrition needs
to identify the enteral formula categories needed.
4
When
available formulas in each category are evaluated, formulas
that will meet the estimated nutrition needs of the patient are
usually preferred to those tailored to specific diagnoses.
5
Evidence-based research can inform the selection of products
and is especially helpful when considering specialty and dis-
ease-specific formulas.
6
Specialty formulas are considerably
more expensive than standard formulas, and research to sup-
port the increased cost may be lacking. Evidence-based guide-
lines from the American Society for Parenteral and Enteral
Nutrition and the Evidence Analysis Library from the
Academy of Nutrition and Dietetics can be utilized to identify
indications and appropriate use for disease-specific formulas.
Although shortages of enteral formulas have not been as
common as recent PN shortages, certain EN formulas may
sometimes be unavailable due to demand, manufacturing
issues, or disaster. By identifying which products have similar
nutrient profiles and indications, the formulary selection com-
mittee can develop a substitutions list to systematically iden-
tify appropriate alternative formulas to use if a shortage
occurs. This can then be implemented and communicated in a
timely manner when needed. The substitutions list can also be
used to select products for patients whose home formula is not
available on the institution’s current formulary.
Question 5.3. How should human breast milk (HBM) be
managed as an enteral formula?
Practice Recommendations
1. Use HBM for infant feeding whenever possible and
when there are no medical contraindications.
2. If maternal human milk is not available, use
pasteurized donor human milk for premature infants.
3. Donor milk should come from an accredited (Human
Milk Banking Association of North America
[HMBANA]) milk bank or commercial company that
uses HMBANA or more stringent guidelines. Do not
purchase HBM from individuals or through the Internet.
4. Develop at the healthcare organizational level policies
for the collection, receiving, storage, labeling, and
feeding of HBM. Storage recommendations are
described in Table 2.
5. The recommended length of time that milk can be
frozen at –20C (–4F) should be shortened to 3 months.
6. HBM should not be preheated for feeding to a
temperature greater than 40C (104F).
7. Use fortified HBM for premature infants.
8. Use sterile products to fortify HBM, whenever possible.
9. Fortify HBM in a milk lab under sterile conditions.
The optimal timing between human milk fortification
and feeding is not known.
10. Educate all mothers expressing HBM regarding
lactation science, as well as human milk collection
and storage, including cleaning of the breast pump.
Rationale
Human milk is the feeding of choice for infants.
7
Use of HBM
offers many benefits to mothers and infants, including prema-
ture infants.
8,9
However, the nutrient profile of unfortified
HBM is not adequate to support the growth of premature
infants; therefore, HBM for premature infants must be
fortified.
8–11

50 Journal of Parenteral and Enteral Nutrition 41(1)
Guidelines for use of HBM from mothers who abuse
drugs. The Academy of Breastfeeding Medicine and the
American Academy of Pediatrics have guidelines regarding
the use of HBM from mothers who admit to abusing drugs.
12
Milk from adequately nourished mothers who are HIV nega-
tive, who have had consistent prenatal care, and who are par-
ticipating in a treatment program can be used.
12
Use of donor human milk. If maternal HBM is unavailable,
the use of donor HBM is recommended for premature infants
by the American Academy of Pediatrics and the European
Society for Pediatric Gastroenterology, Hepatology, and
Nutrition.
7,13
Because the protein content of donor HBM
depends on the stage of lactation, various fortification strate-
gies may be needed to ensure the protein content of all donor
HBM is sufficient.
14,15
Organizations can acquire donor milk
from an accredited Human Milk Banking Association of
North America (HMBANA) human milk bank or a commer-
cial company that uses similar stringent donor selection and
HBM preparation guidelines. Buying HBM from the Internet
is not safe.
16
The U.S. Food and Drug Administration recom-
mends against feeding infants HBM acquired directly from
individuals or through the Internet.
17
Fortification of human milk. Powdered products can never
be completely sterile. Therefore, it is recommended that liquid
sterile products be used to fortify HBM whenever possible.
15
It is best to fortify HBM away from the bedside, in a sterile
milk lab. The optimal time between HBM fortification and
feeding is not known. It is suggested that this time be as short
as feasible to limit the breakdown of nutrients in HBM. Arti-
cles using prior renditions of the current human milk fortifiers
reported an increase in osmolarity over time.
18,19
Human milk storage and handling. The Academy of Nutri-
tion and Dietetics published recommendations for HBM stor-
age for hospitalized infants in 2011.
20
More recent literature
raises concerns about long-term freezing of unpasteurized
HBM at –20C (–4F).
21
The dornic activity is a measure of
the acidity of HBM and is used as an indirect method of
assessing milk quality and bacterial contamination.
21
Lipopro-
tein lipase maintains its activity at this temperature, and this
activity increases when HBM is frozen for more than 3
months, which is thought to result in a breakdown of triglyc-
erides to free fatty acids that could damage the intestinal epi-
thelial cells.
22
Slutzah and colleagues
23
have recommended that fresh
HBM can be refrigerated for up to 96 hours; however, their
study was not conducted in a real-time environment with
multiple entries of HBM into the same bottle. According to
the Academy of Nutrition and Dietetics recommendations,
refrigeration for 96 hours is acceptable with unit-dosed,
single-entry access.
20
In a unit with multiple entries, it
seems reasonable to be more conservative about refrigera-
tion storage times, limiting refrigerated storage to 72 hours.
In 2015, Bransburg-Zachary and colleagues
24
raised concern
about the heating of HBM for infant feeding. HMBANA advo-
cates for the warming of human milk for premature infants to
body temperature.
25
Term infants may have milk directly from
the refrigerator or at room or body temperature.
25
At tempera-
tures greater than 40C (104F), the nutritional and immuno-
logical properties of HBM begin to deteriorate. The amount of
time that HBM is kept warm is also important; at 38C
(100.4F), lipolysis is rapid with a 440% increase in free fatty
acids in an hour.
26
Published reports of infants becoming ill as a result of HBM
contamination are few; however, contamination can be a prob-
lem. HBM expressed using breast pumps has a higher rate of
contamination than HBM expressed by manual expression.
27
Educational intervention may decrease the prevalence of
contamination.
Question 5.4. What are the best ways to determine
clinical advantages/disadvantages of the closed EN
system?
Table 2. Recommendations for Human Breast Milk Storage for Hospitalized Infants.
Storage Method and Temperature Recommended Storage Time
Freezer (home combined with refrigerator) 3 months; new evidence would suggest shortening this time
Freezer (–20°C, –4°F) 6–12 months; new evidence would suggest reducing this to 3 months
Freezer (–70°C, –94°F) >12 months
Refrigerator (4°C, 40°F), fresh milk New evidence would suggest lengthening this from 48 to 72 hours unit
dosed, single entry 96 hours
Refrigerator (4°C, 40°F), thawed milk 24 hours
Refrigerator (4°C, 40°F), fortified milk 24 hours
Refrigerator (4°C, 40°F), thawed pasteurized donor milk 48 hours
Cooler with ice packs (15°C, 59°F) fresh milk 24 hours
Room temperature (25°C, 77°F) <4 hours
Adapted with permission from Lessen R, Sapsford A. Expressed human milk. In: Robbins ST, Meyers R. Infant Feedings: Guidelines for Preparation of
Human Milk and Formula in Health Care Facilities. Chicago, IL: American Dietetic Association; 2011:47.

Boullata et al 51
Practice Recommendations
1. Select an open or closed system for EN delivery based
on the following factors of each system and the needs
of the institution:
a. Cost: The use of a closed system can potentially
save money because it requires fewer nursing
resources and lowers the risk of infections due to
bacterial contamination.
b. Safety: If an open system is used, facilities must
be willing and able to implement protocols and
diligently monitor compliance with all EN product
handling and administration procedures, including
hand hygiene, proper handling of enteral feedings
and sets, and hang-time limits.
Rationale
Over the years, many healthcare institutions have transitioned
from open enteral systems (in tetra-packs, bottles, or cans) to
closed enteral systems (in bags or rigid containers) in efforts
to reduce infection from contaminated enteral formulas and
to reduce nursing time. Commercially available liquid EN
products are sterilized before distribution but can become
contaminated when used at the facility. Contamination of
enteral formulas can cause abdominal distension,
28
diar-
rhea,
29–31
and bacteremia following administration.
32
Several
studies have shown that the risk of contamination is greater
with open systems because these systems increase physical
handling of EN.
33–37
Closed systems can decrease manipula-
tion and human contact with enteral formulas and feeding
administration sets, which in turn reduces the risk of contami-
nation.
38–43
However, some studies have shown that open sys-
tems can be safely used when staff practice good hygiene and
comply with proper handling procedures.
44–46
Multiple stud-
ies have demonstrated that using a closed system reduces
nursing time.
46–48
Closed systems can be costly because of formula packag-
ing and waste from unused formula (closed system products
come in 1000-mL or 1500-mL containers, whereas open-sys-
tem products come in 237-mL or 250-mL containers). Closed
containers have an increased hang time of up to 48 hours
(compared to 4–8 hours with open systems); however, most
closed containers are discarded after 24 hours due to current
manufacturer recommendations to change enteral feeding
sets every 24 hours and to spike each closed container only
once.
49
Nevertheless, studies have found that using closed
systems with increased hang times reduces waste and
costs.
49,50
A 2013 cost-analysis study showed that a closed
system was more expensive than an open system when
accounting for waste ($4.80 per patient day compared to
$4.21 per patient day).
49
However, when nursing time was
factored into the costs, the expense of the open system
increased to $9.83 per patient day.
Pediatric Open Systems
Open systems will likely need to continue to be utilized in the
pediatric population because many products are only available
in powdered form. Powdered infant formulas are not sterile upon
manufacture. In 2004, an infant died as a result of a Cronobacter,
formerly called Enterobacter sakazakii, infection that was found
in the infant’s reconstituted powdered infant formula.
51
The
organism was also found in unopened cans of the formula.
Ready-to-feed and concentrated liquids are sterile products, but
not all formulas come in this form as noted above. Therefore, it
is recommended that powdered formula not be used for immune-
compromised infants, if other options are available.
Over time, infant formula manufacturers have converted
many products, such as human milk fortifiers, from powder to
liquid forms. However, certain products are only available in
powder, such as products for infants with inborn errors of
metabolism, infant and pediatric elemental formulas, and a
specialty infant renal formula. Some formulas only come as
ready-to-feed or powder products and are not supplied in con-
centrated liquid form. If the clinician wants to use these formu-
las at a higher calorie density, nonsterile powder is commonly
added to ready-to-feed formula, which increases the risk of
contamination.
HBM is the preferred nutrition for infants. If mother’s own
milk is not available, donor human milk may be used. Donor
milk is pasteurized, which diminishes the immunoprotective
nutrients. Compared to fresh or frozen HBM, proliferation of
bacterial pathogens in pasteurized HBM was 1.8–4.6 times.
52
In 2011, the Academy of Nutrition and Dietetics issued
guidelines for hang times for infant feedings,
53
and these strin-
gent guidelines are recommended for neonates and immuno-
compromised infants until there is sufficient further evidence.
In a prospective, descriptive study of 30 pediatric patients,
Lyman et al
44
found that “decanted enteral formula adminis-
tered continuously over 12 hours in a pediatric hospital setting
has a lower than expected rate of bacterial growth when recom-
mended handling practices are followed.” This evidence might
influence the Academy of Nutrition and Dietetics to revise the
hang-time guidelines to 12 hours for pediatrics; however, there
is no evidence at this time that guidelines for immunocompro-
mised or neonatal patients should be altered.
44
Question 5.5. What are the critical elements of the EN
order that need to be transmitted to ensure safe
product preparation?
Practice Recommendations
1. Develop and design standardized EN orders (CPOE or
editable electronic templates, or paper as a last resort)
for adult and pediatric EN regimens to aid prescribers
in meeting each patient’s nutrition needs and to
improve order clarity.

52 Journal of Parenteral and Enteral Nutrition 41(1)
2. Include all critical elements in the EN orders: (1)
patient identifiers, (2) the formula name, (3) the EAD
site/device, (4) the administration method and rate,
plus (5) water flush type, volume, and frequency.
Incorporate the feeding advancement order, transitional
orders, and implementation of complementary orders
into protocols. All elements of the EN order must be
completed when EN is modified or reordered.
3. Avoid the use of unapproved abbreviations or
inappropriate numerical expressions.
4. Encourage the use of generic terms to describe EN
formulas. All elements of the EN order must be
completed when EN is modified or reordered.
5. Provide clear instructions related to modular products,
including product dose, administration method, rate,
and frequency.
6. Establish and enforce policies and procedures that clearly
describe the preparation of powdered EN products,
including who will evaluate compatibility, measure the
dose, reconstitute the product, what diluent and source
will be used, the location of preparation, labeling
including beyond use date and time, and storage.
Rationale
Many problems associated with EN orders often result in
inadequate delivery of formula to patients in critical care set-
tings. These problems are attributed to underordering, fre-
quent cessation of the enteral infusion, and slow advancement
of the EN to goal rate.
54,55
EN protocols,
54,56–58
algorithms,
59
and clinical practice guidelines
60
have been developed to
standardize enteral feeding practice, and many have resulted
in an improvement in the delivery of enteral feedings to
patients. One group developed a protocol that standardized
ordering, nursing procedures, and rate advancement and also
limited interruptions to EN administration. Use of the proto-
col improved delivery of goal volumes, although there was
physician resistance to using a standard order.
55
A Canadian
group improved delivery of the required formula volume
using a protocol.
56
Woien and Bjork
59
reported on a feeding
algorithm that was developed to increase the likelihood of
meeting nutrition requirements in intensive care. The algo-
rithm also resulted in an increased utilization of EN (rather
than PN) and in the number of patients who met EN adminis-
tration goals. Another study described a stepwise process to
develop and implement a tailored action plan that could be
adopted in ICUs with differing characteristic and used to help
identify barriers to adequate provision of EN in critically ill
patients (eg, EN formula and feeding pump availability on
units, use of a protocol to reduce interruptions, an algorithm
for managing diarrhea) and help those facilities tailor inter-
ventions to improve nutrition practice.
61
Patient-specific EN orders should include all critical ele-
ments: (1) patient demographics, (2) the formula name, (3)
delivery site and access device, and (4) administration method
and rate, plus water flush type, volume, and frequency. Orders
can be provided as a single order representing a specific pre-
scription, or they can be part of a larger protocol that directs
advancement of EN from initiation to a goal rate or volume
that represents a nutritionally adequate end point. Specific
preparation or administration instructions can also be included
in these protocols. Such instructions are especially important
for safe use of modular products or reconstituted powdered
products to meet patient requirements. The inclusion of transi-
tional orders will direct weaning from EN, and ancillary orders
may address various patient care issues. Orders may be com-
municated through a CPOE system or via editable templates in
electronic format, with paper forms clearly being a last resort
or for when electronic systems are down.
Patient identifiers: The order should clearly state the
patient’s name, date of birth, location, and medical record
number (MRN).
Formula: The formula should be clearly identified in the
order by a generic name as well as by the specific product brand
depending on institutional policy. For example: A formula that
contains 1 calorie per mL can be generically identified as “iso-
tonic” or “standard”; formula that contains 2 calories per mL
can be generically identified as “calorie dense”; a partially
hydrolyzed formula can be generically identified as “semi-ele-
mental” or “peptide based.” Formula orders may also include
the administration of modular products used to enhance the pro-
tein, carbohydrate, fat, or fiber content of the enteral regimen.
In the adult population, these products are usually administered
directly to the patient via the EAD in prescribed amounts and
frequency with specific administration guidelines but are most
often not added to the enteral formula. In the neonatal and pedi-
atric population, fluid tolerance limits are a greater concern;
therefore, the base formula is often augmented with a modular
macronutrient as compatibility allows. When this type of
manipulation to infant formula is prescribed, the base formula,
the modular product, and the base and final concentration of
formula per 100 calories are all considered.
62,63
If this is done in
the home, it is important to teach the parents or caregivers the
proper method to prepare a formula with additives.
Delivery site/device: The route of delivery as well as the
access device for EN formula administration should be clearly
identified in the order to prevent wrong-site administration.
Enteral misconnections have been reported in the literature.
64
Identification of the infusion site (eg, jejunal port of gastrojeju-
nostomy tube) also decreases the chance of inadvertent use of
the wrong feeding port for enteral infusion.
Administration method and rate: Bolus, gravity, or continu-
ous method (rate based or volume based): volume or rate of
administration and timing of formula delivery within a speci-
fied period of time (24 hours or cyclic) should be clearly set
forth in an EN order.
Supplementary orders: Orders that differ from the standard
formula rate, route, and volume prescriptions. These can include:

Boullata et al 53
Advancement orders: These orders direct the progression of
an EN regimen from initiation through to an end point or goal
formula volume infused over a specified time period. Increases
in formula volume or rate of administration to achieve a goal
should be clearly written. Protocols should visibly illustrate
feeding adjustments when volume based feeds are utilized.
Advancement orders also need to be coordinated with decreases
in PN.
65
Transitional orders: The incremental decreases in formula
volume over a period of time to accommodate for an increase
in oral intake.
Ancillary orders: Routine or ancillary orders will depend
on both the population and setting. These orders are based
on institutional policies for care of the enterally fed patient,
such as orders for HOB elevation, tube occlusion treat-
ment, bowel management,
66
and monitoring laboratory
parameters.
EN orders contain all the elements that should be part of
an EN order plus suggestions for ancillary and transitional
orders. Many institutional settings already utilize CPOE
systems, and these systems should be designed with detailed
order sets that promote safety by using EHR drop-down
menus within each element of an EN order, including
required fields. Such menus may facilitate standardized
advancement of initial administrations to goal volumes, uni-
form enteral access device flushing volumes and methods,
and population-specific ancillary orders. Orders for moni-
toring, flushing, and transitioning from tube feeding can
also be included.
Question 5.6. What are the minimum requirements for
the safe preparation of EN formulas that need to be
decanted from small commercial containers or
reconstituted from dry powder?
Practice Recommendations
1. Use competent personnel trained to follow strict
aseptic technique for formula preparation.
2. Immediately refrigerate formulas reconstituted in
advance. Discard unused reconstituted and refrigerated
formulas within 24 hours of preparation.
3. Expose reconstituted formulas to room temperature for
no longer than 4 hours. Discard unused formula after
this time.
4. Use a sterile water source for formula reconstitution.
5. Use formula decanted from a screw cap instead of a
flip top.
Rationale
Between 0% and 57% of enteral formulas prepared in the hos-
pital and over 80% of those prepared in the home have been
found to be contaminated with bacteria.
39,67–69
EN preparation
may include the mixing, reconstitution, or dilution of modular
products and formula with sterile water, and/or pouring the
formula into an administration container. The sterility of the
commercially available liquid EN products, as well as that of
the sterile bags and administration sets, is disrupted by any
manipulation, which increases the risk for contamination.
Commercially available EN products manufactured in dry
powder form are not required to be sterile and may be con-
taminated by the end of the production process prior to reach-
ing the market. A study of powdered infant formulas across
several European countries revealed Enterobacter species
contamination in 53% of 141 samples.
70
Although these bacte-
ria were found in amounts within the accepted maximal limits,
the organism would be expected to multiply rapidly once
these products are reconstituted with water, especially if at
room temperature.
71
A more recent study of EN powder for-
mulas in the care of adults identified considerable contamina-
tion. Out of 28 samples of reconstituted powdered formulas,
27 (96%) had total viable bacterial counts greater than 10
3
colony-forming units (CFU)/g.
71
The CDC recommends that
if a powder EN product is selected to meet a patient’s needs,
trained personnel should prepare it following strict aseptic
technique.
72
Reconstituted formula exposed to room tempera-
ture for more than 4 hours should be discarded. In addition,
the reconstituted formula that is not immediately used must be
promptly refrigerated, and any formula that remains 24 hours
after preparation must be discarded. In the absence of a for-
mula preparation room, the pharmacy can support reconstitu-
tion of powdered formula in a laminar airflow environment.
The water supply may be a source of potential contamina-
tion if purified water is not used. All water supplied for feeding
preparation must at least meet federal standards for drinking
water and not contain contaminants. For reconstitution of pedi-
atric and neonatal formulas, the water needs to be sterile.
53,72
This should also be considered for reconstituting formulas
intended for adults. Weenk et al
35
compared various feeding
systems and found a sterile glass bottle containing enteral for-
mula to be associated with the lowest level of microbial growth
from touch contamination. They also found that decanted for-
mula poured from a container with a screw cap into a feeding
bag was associated with lower levels of microbial growth than
formula poured from a container with a flip top (similar to the
type of top found on a soda can).
35
Question 5.7. What are the safety issues when using
blenderized tube feedings and how can the risk of
complications be reduced?
Practice Recommendations
1. Prepare blenderized tube feedings (BTF) using safe
food-handling techniques, and store it at refrigerator
temperature immediately after preparation. Discard
any unused portion after 24 hours.

54 Journal of Parenteral and Enteral Nutrition 41(1)
2. Limit the hang time of blenderized tube feedings
(BTF) to 2 hours or less.
3. Give BTF only via a gastrostomy tube that is 14 Fr in
size or greater.
4. Do not use BTF in patients who do not have a proven
tolerance to bolus feeds, those who are medically
unstable, or those who lack a mature gastrostomy site
that is free of infection.
5. Involve a registered dietitian or nutrition support
clinician in the development of the BTF formula to
ensure adequate nutrient delivery.
6. Sanitize mechanical devices (eg, blenders) used to
prepare BTF after each use with an established protocol.
Rationale
An alternative to commercial enteral formulas, BTFs use foods
that are blended to a consistency that allows for ease of use
with a feeding tube.
73
BTFs can be provided exclusively or in
conjunction with a commercial formula. In addition, commer-
cially prepared, ready-to-use, real-food blenderized formulas
are available for those patients who do not want to make their
own homemade formulas.
There is limited research on the safety and efficacy of BTF
in home-fed patients. Several studies demonstrate some benefit
with this technique in, for example, postfundoplication
patients. However, more research is needed to demonstrate the
benefit in additional patient populations generally maintained
on partial or complete home nutrition support.
74,75
Home-prepared BTFs have a higher risk of cross-contami-
nation and potential for foodborne illness than commercial EN
products.
76–78
High risk of contamination was a major reason
why institutions moved away from using BTF in the hospital
setting when commercial enteral formulas became available. In
the home environment, care should be taken to prepare BTFs
using safe food-handling techniques to prevent cross-contami-
nation. Once prepared, the BTF should be immediately used or
immediately refrigerated at appropriate temperatures.
73,79
Access to adequate refrigeration, clean water, and electricity is
imperative before considering a change to BTF.
80
Given the
potential for infection associated with foodborne illness, use of
BTF may not be appropriate among medically unstable patients,
immunocompromised patients, or those without a mature feed-
ing tube site.
73,81
BTF should not be held at room temperature
for more than 2 hours due to concerns about food safety and
bacterial contamination; therefore, a bolus regimen instead of a
continuous infusion is recommended.
73,76
Patients with volume
limitations or known intolerance to bolus feeds are not good
candidates for BTFs. Refrigerated BTF formula that is not used
within 24 hours of formulation should be discarded.
There may be an increased risk of tube occlusion with BTFs
given their high viscosity. Therefore, BTFs are not recom-
mended for patients with a feeding tube smaller than 14 French
as smaller tubes are more likely to occlude.
75
A recent study was
conducted to determine the flow rate of BTFs through the new
enteral (ENFit) connector system compared to various other
available feeding tube components. In this study, ENFit and
Cath-tip syringes flow and pressure requirements were essen-
tially equivalent. If BTFs can go through the Cath-tip syringe,
they should also be able to go through the ENFit connector.
82
Another study by Mundi et al
83
observed a need for increased
force with the ENFit connector to administer blenderized for-
mulas compared to traditional connectors, but this study was
conducted with device prototypes and not with FDA-approved
products. Currently, the FDA and other independent labs are
conducting flow and pressure studies with a variety of tubes and
a variety of formulas, including blenderized diets.
Several studies have demonstrated that the macronutrient and
micronutrient content of BTFs is highly variable and the energy
content is often overestimated.
76,78,83–85
Registered dietitians
should be involved in development of the BTF composition to
ensure adequate nutrient delivery in the home environment and
help maintain consistency of the regimen to prevent
underfeeding.
74,76,86
Questions 5.8–5.10. Does a standardized approach to
labeling EN reduce errors and what are the critical
elements of the EN order that need to appear on the
patient-specific label? What elements on a commer-
cial container must be present to meet the critical
elements of the EN order/patient identification?
How does one best avoid errors associated with
sound-alike, look-alike product names and labels?
Practice Recommendations
1. Include all the critical elements of the EN order on the
EN label: patient identifiers, formula type, enteral
delivery site (route and access), administration method
and type, and volume and frequency of water flushes.
2. Standardize the labels for all EN formula containers,
bags, or syringes to include who prepared the formula,
date/time it was prepared, and date and time it was
started.
3. Express clearly and accurately on all EN labels in any
healthcare environment what the patient was ordered.
Given changes to administration rates/volumes,
consider patient-specific labels that state:
a. “Rate not to exceed ______”
b. “Volume not to exceed _______”
4. Include on the label of HBM stored in the hospital:
contents in container, infant’s name, infant’s medical
record number, date and time of milk expressed, maternal
medications, fortifiers added, and energy density.
5. State on the HBM label whether the milk is fresh or
frozen, date and time the milk was thawed, and the
appropriate expiration date. Bar codes, special colors,
or symbols may be used to further identify the HBM.

Boullata et al 55
6. Label commercial enteral containers “Not for IV Use”
to help decrease the risk for an enteral misconnection.
7. Carefully check commercial enteral container labeling
against the prescriber’s order. Be aware of sound-alike
or look-alike product names that may be mixed up on
the order or during selection of the product.
Rationale
In any healthcare environment, patient-specific, standardized
labels for EN express clearly and accurately what the patient is
receiving at any time. Having standardized components on a
label decreases potential confusion when a patient is trans-
ferred to a different unit within a facility or when a new staff
member takes over a patient’s care.
87
Clear labeling that the
container is “Not for IV Use” helps decrease the risk for an
enteral misconnection. Proper labeling also allows for a final
check of that enteral formula against the prescriber’s order.
88
Standardized labels can be affixed to all EN formula admin-
istration containers (bags, bottles, syringes used in syringe
pump). Each label lists the 4 critical elements of the EN order:
patient identifiers, formula type, enteral delivery site (route and
access), and administration method (see Table 3). It also identi-
fies the individuals responsible for preparing and hanging the
formula as well as the time and date the formula is prepared and
hung.
88,89
See Figures 5 through 8 for examples of labels, which
may also include nutrient information if the label is computer
generated. Care should be taken in developing a label that is
clear and concise and of a size that fits neatly on the container.
Special considerations regarding the labeling of HBM. Clear
and concise labeling of HBM is essential to prevent errors in the
delivery of HBM to the infant. The label of milk stored in the
hospital should include the following information: contents in
container (HBM), the infant’s name, the infant’s medical record
number, the date and time when milk was expressed, maternal
medications, fortifiers added to the HBM, and the energy den-
sity of the HBM.
90
Additionally, the label should state whether
the milk is fresh or frozen, date and time the milk was thawed,
and expiration date based on whether milk is fresh or frozen.
53
If the mother is separating fore and hind milk, this designation
should appear on the label. Unique identifiers may be used to
describe other factors such as colostrum, transitional, and
mature milk. Bar codes, special colors, or symbols may be used
to further identify the HBM. Hospitals may use computer-gen-
erated or, at last resort, handwritten labels (see Figures 7 and 8).
Topics for Future Research
Efficacy of methods and objectives for developing EN
formularies
Best practice for formulary decision-making process
The cost-effectiveness of including specialty formulas
in formularies
The optimal size of formularies
The costs and benefits of participating in corporate-
buying organizations
Safe storage and hang times for all categories of human
milk, including the concern for the dornic activity of
unpasteurized human milk during freezing
The optimal feeding temperature for HBM for
premature infants to promote digestion without altering
the beneficial properties in human milk and the length
of time HBM can safely remain at this temperature
The optimal time between preparation and feeding the
infant using the newer HBM fortifiers and modular
additives
Table 3. Components of the Formula Label.
Labeling of Enteral Formula Labeling of Incoming Human Breast Milk
Patient’s name
Medical record ID number
Formula name and strength of formula, if diluted
Date and time formula prepared
a
Date and time formula hung
a
Administration route
Rate of administration expressed as mL/h over 24 hours if
continuous administration or “Rate not to exceed ______”
or “Volume not to exceed _______”
Administration duration and rates are to be expressed on the
label if the EN is cycled or intermittent
Initials of who prepared, hung, and checked the EN against
the order.
Appropriate hang time (expiration date and time)
Dosing weight if appropriate
“Not for IV Use”
Infant’s name
Medical record ID number
Dosing weight
Date and time that milk expressed
Medication or supplements being taken by the mother
Specify whether milk is fresh or frozen
Contents in syringe/container (expressed breast milk)
If frozen, date and time milk thawed
Expiration date (based on whether the milk was fresh or frozen)
“Not for IV Use”
Fortified human breast milk also includes:
{{
Name of fortifier
{{
Final concentration
{{
Date and time formula prepared
{{
Initials of who prepared, hung, and checked the EN against
the order
EN, enteral nutrition; ID, identification; IV intravenous.
a
Date-time formula prepared and date-time formula hung may be different, so note both.

56 Journal of Parenteral and Enteral Nutrition 41(1)
Figure 5. Standard enteral nutrition (EN) label template (adult patient). ID, identification; IV, intravenous. Adapted from Bankhead R,
Boullata J, Brantley S, et al. Enteral nutrition practice recommendations. JPEN J Parenter Enteral Nutr. 2009;33(2):122-167.
Figure 6. Standard enteral nutrition label template (neonatal or pediatric patient). ID, identification; IV, intravenous. Adapted from
Bankhead R, Boullata J, Brantley S, et al. Enteral nutrition practice recommendations. JPEN J Parenter Enteral Nutr. 2009;33(2):122-167.

Boullata et al 57
Ideal fortification for mother’s and donor human milk
for the premature infant in and outside the hospital
Methods to analyze and fortify human milk
Best method of fortification for the infant who requires
surgery or the infant with short bowel syndrome
The safety and cost-effectiveness of the closed system
on patient and nursing satisfaction
References
1. Coffey LM, Car M. Evaluating an enteral nutrition formulary. J Am Diet
Assoc. 1989;89:64-68.
2. Anderson A, Baker G, Carmody M, et al. Partnering to improve the supply
chain. Mater Manag Health Care. 2006;15(2):42-51.
3. Smith CR. Determining when integrated delivery systems should belong
to GPOs. Healthc Financ Manage. 1998;52:38-41.
4. Wildish DE. Enteral formulary management: a cost-effective approach.
Can J Dietetics Pract Res. 2006;67:193-198.
5. Fussell St. Enteral formulations. In: Materese Le, Gottschlich MM.
Contemporary Nutrition Support Practice. 2nd ed. Philadelphia, PA:
Saunders; 2004:188-200.
6. Biesemeir C. Economic considerations in healthcare organizations.
In: Issues and Choices in Clinical Nutrition Practice. Baltimore, MD:
Lippincott Williams and Wilkins; 2007:63-86.
7. American Academy of Pediatrics, Section on Breastfeeding. Breastfeeding
and the use of human milk. Pediatrics. 2012;129:e827-e841.
8. Fallon EM, Nehra D, Potamkin AK, et al. ASPEN clinical guidelines:
nutrition support of neonatal patients at risk for necrotizing enterocolitis.
JPEN J Parenter Enteral Nutr. 2012;36:506-523.
9. Nehra D, Carlson SJ, Fallon SM, et al. ASPEN clinical guidelines: nutri-
tion support of neonatal patients at risk for metabolic bone disease. JPEN
J Parenter Enteral Nutr. 2013;37:570-598.
10. Ziegler EE. Human milk and human milk fortifiers. In: Koletzko B,
Poindexter B, Uuay R, eds. Nutritional Care of Preterm Infants: Scientific
Basis and Practical Guidelines. Basel, Switzerland: Karger; 2014:215-227.
11. Wessel JJ. Human milk. In: Corkins MR, ed. The ASPEN Pediatric
Nutrition Support Core Curriculum. 2nd ed. Silver Spring, MD: ASPEN;
2015:153-168.
Figure 7. Standard human breast milk label template (infant patient). HBM, human breast milk; ID, identification; IV, intravenous.
Adapted from Bankhead R, Boullata J, Brantley S, et al. Enteral nutrition practice recommendations. JPEN J Parenter Enteral Nutr.
2009;33(2):122-167.
Figure 8. Human breast milk storage label. ID, identification.
Reprinted from Bankhead R, Boullata J, Brantley S, et al. Enteral
nutrition practice recommendations. JPEN J Parenter Enteral
Nutr. 2009;33(2):122-167.

58 Journal of Parenteral and Enteral Nutrition 41(1)
12. Jansson LM. ABM clinical protocol #21: guidelines for breastfeeding and
the drug dependent woman. Breastfeed Med. 2009;4:225-228.
13. Arslanoglu S, Corpeleijn W, Moro G, et al. Donor human milk for preterm
infants: current evidence and research directions. J Pediatr Gastroenterol
Nutr. 2013;57:535-542.
14. Valentine CJ, Morrow G, Fernandez S, et al. Docosahexaenoic acid and
amino acid contents in pasteurized donor milk are low for preterm infants.
J Pediatr. 2010;157:906-910.
15. Colaizy TT. Donor human milk for preterm infants. Clin Perinatol.
2014;41:437-450.
16. Geraghty S, McNamara KA, Dillon CE, et al. Buying human milk via the
Internet: just a click away. Breastfeeding Med. 2013;8:474-478.
17. Food and Drug Administration. Use of donor milk. http://www.fda.
gov/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/
ucm235203.htm. Accessed March 2016.
18. De Curtis M, Cansduso M, Pieltain C, et al. Effect of fortification on
the osmolality of human milk. Arch Dis Child Fetal Neonatal Ed.
1999;81:F141-F143.
19. Choi A, Fusch G, Rochow N, et al. Target fortification of breast milk: pre-
dicting the final osmolality of the feeds. PLoS One. 2016;11(2):e0148941.
20. Lessen R, Sapsford A. Expressed human milk. In: Robbins ST, Meyers
R, eds. Infant Feedings: Guidelines for Preparation of Human milk
and Formula in Health Care Facilities. Chicago, IL: American Dietetic
Association; 2011:47.
21. Vazquez-Roman S, Escuder-Vieco D, Garica-Lara NR, et al. Impact of
freezing time on dornic activity in three types of milk: raw donor milk,
mother’s own milk, and pasteurized donor milk [published online January
22, 2016]. Breastfeeding Med.
22. Penn AH, Altshuler AE, Small JW, et al. Effect of digestion and storage
of human milk on free fatty acid concentration and cytotoxicity. J Pediatr
Gastroenterol Nutr. 2014;59:365-373.
23. Slutzah M, Codpilly CN, Potak D, et al. Refrigerator storage of expressed
human milk in the neonatal intensive care unit. J Pediatr. 2010;156:26-28.
24. Bransburg-Zachary S, Virozub A, Mimouni FB. Human milk warming
temperature using a simulation of currently available storage and warming
methods. PLoS One. 2015;10:e1028806.
25. Jones F. Best Practices for Expressing, Storing, and Handling Human
Milk in Hospitals, Homes, and Child Care Settings. 3rd ed. Fort Worth,
TX: Human Milk Banking Association of North America; 2011.
26. Wardell J, Wright A, Bardsley W, et al. Bile salt stimulated lipase and
esterase activity in human milk after collection, storage, and heating:
nutritional implications. Pediatr Res. 1984;18:382-386.
27. Boo NY, Nordiah AJ, Alfizah H, et al. Contamination of breast milk
obtained by manual expression and breast pumps in mothers of very low
birth weight infants. J Hosp Infect. 2001;49:2740281.
28. Freedland CP, Roller RD, Wolfe BM, Flynn NM. Microbial contami-
nation of continuous drip feedings. JPEN J Parenter Enteral Nutr.
1989;13(1):18-22.
29. Anderson KR, Norris DJ, Godfrey LB, Avent CK, Butterworth CE.
Bacterial contamination of tube-feeding formulas. JPEN J Parenter
Enteral Nutr. 1984;8:673-678.
30. Okuma T, Nakamura M, Totake H, Fukunaga Y. Microbial contamination
of enteral feeding formulas and diarrhea. Nutrition. 2000;16: 719-722.
31. Fernandez-Crehuet Navajas M, Jurado Chacon D, Guillen Solvas JF,
Galvez Vargas R. Bacterial contamination of enteral feeds as a possible
risk of nosocomial infection. J Hosp Infect. 1992;21:111-120.
32. Levy J, Van Laethem Y, Verhaegen G, Perpete C, Butzlet JP, Wenzel RP.
Contaminated enteral nutrition solutions as a cause of nosocomial blood-
stream infection: a study using plasmid fingerprinting. JPEN J Parenter
Enteral Nutr. 1989;13(3):228-234.
33. Beattie TK, Anderton A. Microbiological evaluation of four enteral feed-
ing systems which have been deliberately subjected to faulty handling
procedures. J Hosp Infect. 1999;42:11-20.
34. Anderton A, Aidoo KE. Decanting—a source of contamination of enteral
feeds? Clin Nutr. 1990;9:157-162.
35. Weenk GH, Kemen M, Werner HP. Risks of microbiological contamina-
tion of enteral feeds during the setup of enteral feeding systems. J Human
Nutr Diet. 1993;6:307-316.
36. Chan L, Yasmin AH, Ngeow YF, Ong GSY. Evaluation of the bacterio-
logical contamination of a closed feeding system for enteral nutrition. Med
J Malaysia. 1994;49(1):62-67.
37. Patchell CJ, Anderton A, MacDonald A, George RH, Booth IW. Bacterial
contamination of enteral feeds. Arch Dis Child. 1994;70:327-330.
38. Beattie TK, Anderton A. Decanting versus sterile prefilled nutrient con-
tainers—the microbiological risks in enteral feeding. Int J Environ Health
Res. 2001;11:81-93.
39. Bott L, Husson MO, Guimber D, et al. Contamination of gastrostomy
feeding systems in children in a home-based enteral nutrition program. J
Pediatr Gastroenterol Nutr. 2001;33:266-270.
40. Marlon ND, Rupp ME. Infection control issues of enteral feeding systems.
Curr Opin Clin Nutr Metab Care. 2000;3(5):363-366.
41. Vanek V. Closed versus open enteral delivery systems: a quality improve-
ment study. Nutr Clin Pract. 2000;15(5):234-243.
42. Moffitt SK, Gohman SM, Sass KM, Faucher KJ. Clinical and laboratory
evaluation of a closed enteral feeding system under cyclic feeding condi-
tions: a microbial and cost evaluation. Nutrition. 1997;13:622-628.
43. Wagner DR, Elmore MF, Knoll DM. Evaluation of “closed” vs “open”
systems for the delivery of peptide-based enteral diets. JPEN J Parenter
Enteral Nutr. 1994;18(5):453-457.
44. Lyman B, Gebhards S, Hensley C, Roberts C, San Pablo W. Safety of
decanted enteral formula hung for 12 hours in a pediatric setting. Nutr Clin
Pract. 2011;26:451-456.
45. Schroeder P, Fisher D, Volz M, Paloucek J. Microbial contamination
of enteral feeding solutions in a community hospital. JPEN J Parenter
Enteral Nutr. 1983;7(4):364-368.
46. Fagerman KE. Limiting bacterial contamination of enteral nutrient solu-
tions: 6-year history with reduction of contamination at two institutions.
Nutr Clin Pract. 1992;7:31-36.
47. Luther H, Barco K, Chima C, Yowler CJ. Comparative study of two sys-
tems of delivering supplemental protein with standardized tube feedings.
J Burn Care Rehabil. 2003;24:167-172.
48. Silkroski M, Allen F, Storm H. Tube feeding audit reveals hidden costs
and risks of current practice. Nutr Clin Pract. 1998;13:283-290.
49. Phillips W. Economic impact of switching from an open to a closed
enteral nutrition feeding system in an acute care setting. Nutr Clin Pract.
2013;28(4):510-514.
50. Bristol S, Meer M, Bashar A, et al. Financial benefit of closed versus open
enteral system [abstract]. Nutr Clin Pract. 2008;23(2):236-237.
51. Centers for Disease Control and Prevention. Enterobacter sakazakii infec-
tions associated with the use of powdered infant formula—Tennessee,
2001. MMWR. 2002;51:297-300.
52. Akinbi H, Meinzen-Derr J, Auer C, et al. Alterations in the host defense
properties of human milk following prolonged storage or pasteurization. J
Pediatr Gastroenterol Nutr. 2010;51:347-352.
53. Robbins ST, Meyers R. Infant Feedings: Guidelines for Preparation
of Human Milk and Formula in Health Care Facilities. Chicago, IL:
American Dietetic Association; 2011.
54. McClave SA, Sexton LK, Spain DA, et al. Enteral tube feeding in the
intensive care unit: factors impeding adequate delivery. Crit Care Med.
1999;27(7):1252-1256.
55. Spain DA, McClave SA, Sexton LK, et al. Infusion protocol improves
delivery of enteral tube feeding in the critical care unit. JPEN J Parenter
Enteral Nutr. 1999;23(5):288-292.
56. Mackenzie SL, Zygun DA, Whitmore BL, Doig CJ, Hameed SM.
Implementation of a nutrition support protocol increases the propor-
tion of mechanically ventilated patients reaching enteral nutrition tar-
gets in the adult intensive care unit. JPEN J Parenter Enteral Nutrition.
2005;29(2):74-80.
57. Taylor B, Brody R, Denmark R, Southard R, Byham-Gray L. Improving
enteral delivery through the adoption of the “Feed Early Enteral Diet

Boullata et al 59
adequately for Maximum Effect (FEED ME)” protocol in a surgical trauma
ICU: a quality improvement review. Nutr Clin Pract. 2014;29(5):639-648.
58. Ventura AM, Waitzberg DL. Enteral nutrition protocols for critically ill
patients: are they necessary? Nutr Clin Pract. 2015;30(3):351-362.
59. Woien H, Bjork IT. Nutrition of the critically ill patient and effects of
implementing a nutritional support algorithm in ICU. J Clin Nurs.
2006;15(2):168-177.
60. Bourgault AM, Ipe L, Weaver J, Swartz S, O’Dea PJ. Development of
evidence-based guidelines and critical care nurses’ knowledge of enteral
feeding. Crit Care Nurse. 2007;27(4):17-29.
61. Cahill NE, Murch L, Cook D, Heyland DK. Improving the provision of
enteral nutrition in the intensive care unit: a description of a multifac-
eted intervention tailored to overcome local barriers. Nutr Clin Pract.
2014;29(1):110-117.
62. Klein CJ. Nutrient requirements for preterm infant formulas. J Nutr.
2002;132(6)(suppl 1):1395S-1577S.
63. Raiten DJ, Talbot JM, Waters JH. Assessment of nutrient requirements for
infant formulas: LSRO report. J Nutr. 1998;128(11S):2059-2294S.
64. Guenter P, Hicks RW, Simmons D, et al. Enteral feeding misconnec-
tions: a consortium position statement. Jt Comm J Qual Patient Saf.
2008;34(5):285-292.
65. Dervan N, Dowsett J, Gleeson E, Carr S, Corish C. Evaluation of over-
and underfeeding following the introduction of a protocol for weaning
from parenteral to enteral nutrition in the intensive care unit. Nutr Clin
Pract. 2012;27(6):781-787.
66. Racco M. An enteral nutrition protocol to improve efficiency in achieving
nutritional goals. Crit Care Nurse. 2012;32(4):72-75.
67. Roy S, Rigal M, Doit C, et al. Bacterial contamination of enteral nutrition
in a pediatric hospital. J Hosp Infect. 2005;59:311-316.
68. Anderton A, Nwoguh CE, McKune I, Morrison L, Grieg M, Clark B. A com-
parative study of the numbers of bacteria present in enteral feeds prepared
and administered in hospital and the home. J Hosp Infect. 1993;23:43-49.
69. Perry J, Stankorb SM, Salgueiro M. Microbial contamination of enteral
feeding products in thermoneutral and hyperthermal ICU environments.
Nutr Clin Pract. 2014;30:128-133.
70. Muytjens HL, Roelofswillemse H, Jaspar GHJ. Quality of pow-
dered substitutes for breast-milk with regard to members of the family
Enterobacteriaceae. J Clin Microbiol. 1988;26:743-746.
71. Baniardalan M, Sabzghabaee AN, Badri S. Bacterial safety of commercial
and handmade enteral feeds Iranian teaching hospital. Int J Prev Med.
2014;5:604-610.
72. Centers for Disease Control and Prevention. Enterobacter sakazakii infec-
tions associated with the use of powdered infant formula—Tennessee,
2001. MMWR. 2002;51:297-300.
73. Johnson T, Spurlock A, Galloway P. Blenderized formula by gastros-
tomy tube: a case presentation and review of the literature. Top Clin Nutr.
2013;28(1):84-92.
74. Brown BM, Roehl K, Betz M. Enteral nutrition formula selection: current
evidence and implications for practice. Nutr Clin Pract. 2015;30(1): 72-85.
75. Jonkers-Schuitema CF. Basics in clinical nutrition: Diets for enteral nutri-
tion home made diets. Eur J Clin Nutr Metab. 2009;4(4):168-169.
76. Mortensen M. Blenderized tube feeding: clinical perspectives on
homemade tube feeding. PNPG Post Publ Pediatr Nutr Pract Group.
2006;17(1):1-4.
77. Pentiuk S, O’Flaherty T, Santoro K, Willging P, Kaul A. Pureed by gas-
trostomy tube diet improves gagging and retching in children with fundo-
plication. JPEN J Parenter Enteral Nutr. 2011;35(3):375-379.
78. Novak P, Wilson KE, Ausderau K, Cullinane D. The use of blenderized
tube feedings. Infant Child Adolesc Nutr. 2009;1(1):21-23.
79. Jalali M, Sabzghabaee AM, Badri SS, Soltani HA, Maracy MR. Bacterial
contamination of hospital-prepared enteral tube feeding formulas in
Isfahan, Iran. J Res Med Sci. 2009;14(3):149-156.
80. Borghi R, Dutra Araujo T, Airoldi Vieira RI, Theodoro de Souza T,
Waitzberg DL. ILSI Task Force on enteral nutrition; estimated composi-
tion and costs of blenderized diets. Nutr Hosp. 2013;28(6):2033-2038.
81. Moe G. Enteral feeding and infection in the immunocompromised patient.
Nutr Clin Pract. 1991;6(2):55-64.
82. Guenter P, McMichael D. Further updates on blenderized diet use with the
new enteral connectors. Lifeline Letter. 2014;35(4).
83. Mundi MS, Epp L, Hurt RT. Increased force required with proposed stan-
dardized enteral feed connector in blenderized tube feeding [published
online April 18, 2016]. Nutr Clin Pract.
84. Santos VF, Morais TB. Nutritional quality and osmolality of home-made
enteral diets, and follow-up of growth of severely disabled children receiv-
ing home enteral nutrition therapy. J Trop Pediatr. 2010;56(2):127-128.
85. Sullivan MM, Sorreda-Esguerra P, Platon MB, et al. Nutritional analy-
sis of blenderized enteral diets in the Philippines. Asia Pac J Clin Nutr.
2004;13(4):385-391.
86. O’Flaherty T, Santoro K, Pentiuk S. Calculating and preparing a pureed-
by-gastrostomy-tube (PBGT) diet for pediatric patients with retching and
gagging postfundoplication. Infant Child Adolesc Nutr. 2011;3(6): 361-364.
87. Ayers P, Adams S, Boullata J, et al. A.S.P.E.N. parenteral nutrition safety
consensus recommendations. JPEN J Parenter Enteral Nutr. 2014;38:
296-333.
88. Perry AG, Potter PA. Clinical Nursing Skills & Techniques. 8th ed. St
Louis, MO: Mosby; 2014:775.
89. Guenter P. Safe practices for enteral nutrition in critically ill patients. Crit
Care Nurs North Am. 2010;22:197-208.
90. Academy of Nutrition and Dietetics. Pediatric nutrition care manual:
Expression, handling, and storage of human milk. https://www.nutrition
caremanual.org/about-pncm. Accessed June 14, 2015.
Section 6. Administration: General
Background
The administration of EN therapy is a step in the process with
significant potential for error. Errors can stem from incom-
plete evaluation of a patient’s tolerance for enteral feeding
that increases the risk for aspiration or GI complications.
Enteral misconnections, poor positioning, pump misadven-
tures, and contamination can all lead to less than optimal
patient outcomes.
Question 6.1. What system-based measures can be
implemented to enhance the safety of EN administration?
Practice Recommendations
1. Develop policy and procedure documents for evidence-
based practices to standardize the approach to and the
administration of EN in all patient populations.
2. Maintain competency as defined within the
organization to maximize safety of the patient for all
caregivers involved in the administration of EN.
3. Develop and use enteral feeding and related protocols
with order sets and checklists to optimize nutrition
delivery and promote safe and effective practice, from
patient evaluation to pump programming.
4. Initiate and update protocols periodically based on
best evidence, including national guidelines and
recommendations to meet the needs of the specific
patient populations.

60 Journal of Parenteral and Enteral Nutrition 41(1)
5. Monitor performance of EN delivery and related care
and have in-place systems to enhance practice in
terms of efficacy and safety as indicated.
6. Encourage change champions, such as nutrition
support team members, to guide EN practice.
7. Include knowledgeable nurses in decision making for
selection and purchase of EN administration sets,
feeding pumps, and access devices.
8. Commit to adequately staffing patient care units on
which many patients receive EN with nurses having
documented competency in EN administration.
9. Support both the physical and cognitive efforts of
nurses and other caregivers involved in maintaining
safe practices around EN administration. For example:
a. CPOE for EN orders with the full order available
on the nursing medication administration record
b. Bar coding on EN containers and patient-specific
labels
c. Prompts for documentation of essential steps in
administration of EN as well as the care and
monitoring related to feeding tube and EN use
10. Develop and implement interdisciplinary quality
improvement programs, including systematic review
and analysis of administration-related EN errors, then
implement subsequent safeguards to address any
identified errors in the process.
Rationale
A transparent and collaborative approach using guidelines, pro-
tocols, and standardized practice based on best evidence
enhances patient care within the EN process. Guidelines are
published periodically to provide recommendations for practice
based on best available current evidence.
1–3
Although the prac-
tice of EN administration varies widely, protocols can standard-
ize and guide practice toward safety. The benefit of using
protocols to enhance clinical practice has been articulated.
4–8
Heyland et al
9
demonstrated that protocols can significantly
improve nutrition practices. Racco
10
discussed development of
a protocol to help overcome barriers to achieving goal rate and
guide staff in areas such as holding feeding for gastric residual
volume (GRV). Protocol order set included starting EN rate,
energy, protein, and fluid goals as set by the nutrition support
clinician, bowel management program, prokinetic agent use as
indicated, and education of this order set. Data collection
revealed that 23 protocol patients achieved goal rate in one-
third the time of 13 patients who received EN in the usual man-
ner. Patients with elevated GRV reached goal 16 hours sooner
when the protocol was used, and those with elevated GRVs
started on prokinetic agents after 3 elevated GRVs 75% of the
time. In an evidence-based implementation project with pretest-
posttest measures, Kenny and Goodman
11
showed that EN
protocols in a military hospital improved practices, such as
keeping the head of the bed up, medication administration, and
tube-unclogging practices, and also increased provision of fam-
ily education. Institutional protocols can guide practice in areas
such as tube placement verification, hang time and feeding set
changes, monitoring tolerance of EN, and adequacy of EN. A
nurse-driven protocol to assess stool for Clostridium difficile as
appropriate can also be helpful. Protocols may be institution
specific. It is advisable to periodically review protocols and
update them as warranted by new evidence.
Order sets can guide appropriate EN product selection, ini-
tiation rate and progression to goal, delivery route, and admin-
istration method. Additionally, they can prompt safety features
in EN care and monitoring. For example, routine monitoring of
laboratory values could be especially helpful for those at risk
for issues such as refeeding syndrome or hyperglycemia. Order
sets can prompt additional fluid administration and offer guid-
ance for staff in areas such as HOB elevation, residual volume
check, and abdominal assessment. Safety practices and proto-
cols can be embedded in the order set to populate the EHR to
schedule and remind staff of necessary clinical tasks. Elements
of EN ordering that should also be included in the order set
include demographics such as patient identifiers, and body
weight might also be included or readily accessible.
12
Accountability is optimized when the system process iden-
tifies who is responsible for what. Organizations can standard-
ize safety practices for EN, such as those related to decreasing
risk for enteral misconnections
13
:
Tracing tubings and lines with reconnections at
handoffs
Training nonclinical staff to ask a qualified clinician
to reconnect lines instead of attempting reconnection
themselves
Discouraging the modification or adaptation of IVs
or EADs even if the availability of adaptors and
connectors is reduced
Labeling of tubes and connectors
Identification and confirmation of solutions label and
labeling of bags with bold statements in terms of
contents
Identification and minimization of conditions and
practices that contribute to healthcare worker fatigue
and mitigate risk
Purchasing of appropriate, safe equipment that meets
standards and guidelines such as those from American
National Standards Institute/Association for the
Advancement of Medical Instrumentation (ANSI/
AAMI)
Careful evaluation of purchasing decisions by an
interdisciplinary task force
Following manufacturers’ guidelines to promote safe
connections
Assessing barriers to guideline adherence is key to effective and
consistent use of guidelines and protocols. The 2013 update to

Boullata et al 61
the Canadian Critical Care Nutrition Guidelines discusses key
strategies to promote their previous guidelines and explores 5
thematic domains in analyzing barriers as well as offering sys-
tem-level quality improvement interventions.
14
This guidelines
update promotes evaluating and monitoring practice via perfor-
mance improvement strategies to enhance nutrition care and
improve patient outcomes. As noted earlier, Kenny and
Goodman
11
have described the development and implementa-
tion of an evidence-based practice protocol for care of patients
with EN tubes; after these performance improvement interven-
tions, HOB elevation was achieved 100% of the time. Lyerla
and colleagues
15
used a modified interrupted time-series design
to collect data on 43 patients and 33 nurses in a 12-bed critical
care unit. They found that a nursing clinical decision support
system integrated into the electronic flow sheet increased adher-
ence to guidelines. Change champions have been shown to
facilitate change processes to improve care.
11,16
This is a role
that can be played by appropriate staff who take an active inter-
est in and accountability for enhancing practice.
Question 6.2. What are the essential components for
EN administration to include in nursing policies,
procedures, and practices?
Practice Recommendations
1. Define the quality control process for receipt,
distribution, storage, preparation, handling, and
administration of EN products.
2. Use sterile liquid EN formulations in preference to
powdered, reconstituted, or blenderized preparations,
whenever possible.
3. Administer EN by, or under the direct supervision of,
competent personnel as defined by the organization.
The personnel who administer EN will:
a. Either accept the delivery of the EN container
identified with the patient-specific label or select
the product from the unit-based inventory and
places the patient-specific label (depending on the
organizational model).
b. Visually inspect the product or preparation for
damage to the container, altered formula
characteristics, and expiration date limits.
c. Confirm that the EN container with the patient-
specific label reflects what has been ordered by
the prescriber. Verify patient identifiers, product
name, and route (and rate) of administration.
d. Perform proper handwashing prior to entering the
patient care area as well as prior to working with
the feeding administration. Don clean gloves prior
to working with the feeding tube and adminis-
tration set.
e. Use aseptic technique in setting up and connecting the
feeding administration set and related equipment.
For example, use a small clean towel under the
patient feeding tube connection to facilitate a clean
area prior to working with the tube.
f. Verify patient identifiers at the bedside matching
those on the EN label, per institutional protocol,
and verify appropriate patient positioning for
feeding.
g. Trace tubing from point of the enteral access device
that was described in the EN order and confirm that
there has been no dislocation of that device.
h. Position the EN container appropriately for the
patient and set up the administration set, priming
it as indicated.
i. Flush EAD and attach administration set using
aseptic technique. The EN container and
administration set make up the EN “delivery
device” and are attached together until discarded.
j. Cover the end with a clean cap for any
disconnection, such as when the feeding is stopped
and the distal end of the delivery device is
disconnected as for nocturnal or gravity bolus
feeding. If a pump is being used as for continuous
feeding, program it based on the EN order.
k. Base any change to the administration rate on
documented EN orders (including prescribed rates
for advancement or weaning).
l. Do not interrupt feeding administration for routine
care unless specifically ordered (as for medication
administration). If the feeding must be interrupted,
flush the tube to reduce the residue in the tube and
decrease potential for clogging.
m. Ensure that administration of enteral medication
via the EAD is reviewed and approved with
documentation as indicated by a knowledgeable
pharmacist.
n. Document EN processes in the patient’s EHR,
with a second entry for any independent double-
check performed. This includes documentation of
tolerance and administration volumes, including
hourly rates as well as amount of intake, and water
flushes.
Rationale
The purpose of policies and procedures is to ensure that staff
follow a consistent standard of care and quality at all lev-
els.
17
Policy statements guide practice by indicating what is
to be done and by whom. They are often based on institu-
tional protocol. Procedures describe the specific methods for
following policies in practice. When staff understand the
rationale for policy and procedures, they may be more likely
to adhere to protocol and use critical thinking. Issues to
address in policies and procedures related to EN delivery are
listed in Table 4.

62 Journal of Parenteral and Enteral Nutrition 41(1)
Organizations can use a systematic plan to promote the
periodic review of policies and procedures and the updating of
policies and procedures based on relevant and current evidence
as well as best practice for patients in the particular care
setting or organization. By conducting quality or performance
improvement, healthcare organizations can monitor practice
and identify areas for improvement and then implement appro-
priate measures to address the findings. For example, Guenter
19
has discussed areas for potential human error related to EN and
suggested the need for nursing oversight to minimize compli-
cations and enhance practice. Kenny and Goodman
11
describe
the use of change champions to increase nursing knowledge of
procedures and issues related to the environment of care.
Policies and procedures for the ongoing care and routine
assessment of EADs can help with early identification of com-
plications and proper interventions. Policies regarding EAD
care and assessment can cover correct tube placement, muco-
sal and skin surfaces assessment, and infection prevention.
Question 6.3. What are the essential steps in EN
administration to prevent aspiration?
Practice Recommendations
1. Maintain elevation of the HOB to at least 30 or upright
in a chair, unless contraindicated, and then consider
reverse Trendelenberg position.
2. Monitor the patient at least every 4 hours for appropriate
positioning. In pediatrics, it is recommended that
infants under 1 year of age sleep on their back and not
have the head of the bed elevated.
3. Minimize the use of sedatives because airway clearance
is reduced in sedated patients.
4. In patients who have difficulty clearing secretions,
follow instructions from appropriate staff regarding how
to clear secretions (eg, by oral suctioning), especially
prior to lowering of the head of the bed and prior to
extubation.
5. Understand that the method of administration (bolus,
intermittent, continuous) and optimal site (gastric,
small bowel) of EN feeding will depend on the patient
needs, medical conditions, tolerance and goals (eg, if
home use is anticipated), and resources available.
6. Monitor patient status for tolerance using measures
such as assessment for abdominal distention, firmness,
and large gastric residual volume (GRV), feeling of
fullness, or nausea that might lead to gastric reflux.
7. Monitor patients for appropriate feeding tube
placement at least every 4 hours or per institutional
protocol. Monitor visible length of tubing or marking
at tube exit site (naris or stoma) and investigate
placement when a deviation is noted.
8. Monitor tube placement and abdominal distention,
firmness for stable patients with longstanding EN therapy.
9. Place infants under 1 year of age on their back for sleep
and do not have the HOB elevated.
Rationale
Aspiration may be related to oral pharyngeal secretions and/
or reflux of esophageal and gastric content, including EN.
Critically ill patients and patients with impaired swallowing
Table 4. Issues to Cover in Policies and Procedures for EN Delivery.
How feeding tubes are to be inserted
How verification of EAD placement is to occur and how EAD placement is monitored
Care for enteral feeding tubes
How to prevent or handle practice challenges such as tube dislodgement
Elements necessary in a provider order for EN
Administration of EN in terms of formula attainment and verification, labeling, administration route, and method (eg, pump use or
gravity bolus feeding method)
Rate or frequency of feedings
Type, volume, and frequency of water flushes
Hang times and equipment handling (eg, in terms of administration set changes)
Medication delivery issues that involve or relate to EN or the enteral tube
Issues related to safety in administration such as recommendations from The Joint Commission Sentinel Event Alert 53 and other
safety issues such as head of bed elevation
18
How to optimize that the appropriate volumes of feeding product and fluid are actually delivered
Methods to monitor for adequacy of EN as well as potential adverse effects, and identify who is responsible for overall and specific
aspects of monitoring as well as patient/family education, especially when transition to the home setting when continued feeding is
anticipated
Frequency of residual assessment, what tubes are to be assessed, how assessment should be performed, and the rationale for the
assessment (the rationale helps staff identify the need for abdominal and more global patient assessment as a guide for tolerance to
EN instead of relying solely on gastric residual assessment)
EAD, enteral access device; EN, enteral nutrition.

Boullata et al 63
may have difficulty protecting their airways. Frequent, good
oral care and oropharyngeal suctioning, especially prior to
lowering the HOB as for positioning, can reduce adverse
events related to aspiration of oropharyngeal secretions.
20,21
Metheny and colleagues
22
compared usual care with an aspi-
ration risk reduction protocol (ARRP), which included HOB
30 or higher unless contraindicated; distal small bowel feed-
ing tube placement, when indicated; and use of an algorith-
mic approach for high GRVs. With usual care, 88% of patients
aspirated compared to 39% with the ARRP protocol. In the
usual care group, 48% of patients developed pneumonia vs
19% in the ARRP group. The authors concluded that combin-
ing HOB at least 30 and use of small bowel feeding site can
reduce aspiration and aspiration-related pneumonia dramati-
cally in critically ill, tube-fed patients. In an earlier article
(2006), Metheny
23
reported that 25 of 201 critically ill
patients had malpositioned enteral feeding tubes and signifi-
cantly higher risk for aspiration than those with tubes appro-
priately positioned. Risk for aspiration may be increased with
enteral tube ports in the esophagus, especially if there are
other risk factors for regurgitation. Some standard NG tubes
(when used to deliver EN for short-term use) have end holes
spaced 3 inches apart, and the standard tube placement mea-
surement of nose to ear lobe to tip of xiphoid (NEX) may be
suboptimal in guiding gastric tube tip placement. A nose to
earlobe to mid-umbilicus (NEMU) method to estimate appro-
priate nasogastric tube placement has been recommended to
promote placement of the tube end holes in or closer to the
gastric fluid pool.
24–26
Appropriate location of the enteral
tube’s distal end must be ascertained prior to instillation of
fluid or medication. It is recommended in infants aged 1 year
or less that they sleep on their back and not have the HOB
elevated. These recommendations are part of the American
Academy of Pediatrics Safe Sleep Initiative, to reduce sudden
infant death syndrome.
27
It is important to obtain, ascertain, and maintain optimal
enteral tube placement to help reduce potential reflux of EN.
Metheny et al
28
performed a retrospective analysis of 428
critically ill, mechanically ventilated patients and found that
the percentage of aspiration was 11.6% lower when feeding
tubes were in the first portion of the duodenum, 13.2% lower
in the second/third portion, and 18% lower in the fourth por-
tion of the duodenum or lower (P < .001). In a randomized
controlled trial of 33 ventilated patients randomized to gastric
vs transpyloric feeding, Heyland et al
29
found that feeding
beyond the pylorus was associated with significant reduction
in gastroesophageal regurgitation and there was a trend
toward less micro-aspiration. In critically ill patients, small
bowel feeding may be associated with less pneumonia than
gastric feeding, but without differences in mortality or days
on a ventilator.
The American Association of Critical-Care Nurses recom-
mend the following to reduce the risk for aspiration: maintain
the HOB 30–45 unless contraindicated; use sedatives as
sparingly as possible; assess feeding tube placement at 4-hour
intervals; observe for change in amount of external length of
the tube; assess for gastrointestinal intolerance at 4-hour inter-
vals; assess residual volume, patient, and abdominal status and
advance the tube if indicated; avoid bolus feeding for those at
high risk for aspiration; assess swallow before oral feedings
are started for recently extubated patients after prolonged intu-
bation; maintain endotracheal tube cuff pressure at an appro-
priate level; and ensure that secretions are cleared from above
the cuff before it is deflated.
2
Question 6.4. Can EN be administered safely in patients
who require prone positioning?
Practice Recommendations
1. Assist the patient in clearing secretions as indicated
and promote good oral hygiene.
2. Assess abdominal status every 4 hours and as indicated
and monitor bowel status as a guide for GI motility
status.
3. Consider short-term use of prokinetic agents if
indicated clinically.
4. Consider transpyloric tube placement for patients who
are at increased risk for aspiration or have persistently
elevated GRVs.
Rationale
Evidence is limited, demonstrating the safety and tolerability
of EN in the prone position, although the minimal available
evidence does not suggest a substantial increase in complica-
tions compared to EN administered in a supine position.
Strategies to increase enteral feeding tolerance in the supine
position such as HOB elevation, small bowel feeding, and use
of prokinetic agents may increase EN tolerance for patients in
the prone position. When the patient’s clinical situation
favors positioning other than HOB elevation at 30 or greater,
as in proned patients, the use of small bowel feeding and pro-
kinetic agents with 25 HOB elevation has been shown to
increase volume tolerance and progress toward feeding
goals.
30
Linn et al
30
reviewed the literature related to administration
of EN in adult patients in the prone position. Only 2 of the 4
studies that they found that met their inclusion criteria were
designed to compare outcomes associated with EN adminis-
tered in the prone vs supine position. The conclusions of these
2 studies were that GRVs of patients in the prone position were
similar to those noted in patients in the supine position; also,
EN delivered to prone-positioned patients did not appear to
increase risk of vomiting or pneumonia in the 2 studies where
this risk was specifically explored. The limited evidence in this
area is highlighted by these authors. Fineman and colleagues
31
compared 51 prone and 51 supine pediatric patients with acute

64 Journal of Parenteral and Enteral Nutrition 41(1)
lung injury in terms of mechanical ventilation, airway manage-
ment, and pain and sedation management, as well as EN. These
authors determined that there was no difference in feeding
complications between the supine and prone positions. They
also noted that patients who were fed via the jejunal route
reached feeding goal earlier than those fed via the gastric route;
however, the study design monitored adverse effects as
opposed to actively looking at outcomes.
Prokinetic agents (eg, erythromycin) and HOB elevation
of 25 were specifically employed in prone patients who
exhibited volume intolerance.
30
Delayed gastric emptying is
reported in 50%–60% of critical care patients, and multiple
factors, including use of vasopressors, and endogenous and
exogenous catecholamines, can contribute to the delay. The
efficacy of erythromycin as a prokinetic agent exceeds that of
metoclopramide, although the effectiveness of erythromycin
diminishes over time. Both agents may have a synergistic
effect when combined. When the use of small bowel feeding
tubes is feasible, it also may increase EN tolerance in prone
patients.
The National Pressure Ulcer Advisory Panel (NPUAP)
recommends limiting HOB elevation to 30 for an individual
on bedrest, unless contraindicated by the patient’s medical
condition or feeding and digestive considerations. NPUAP also
recommends that an individual not be positioned directly on a
pressure ulcer.
32
Schallom and colleagues
33
have compared
research to prevent aspiration and pressure ulcers in critically
ill patients and suggest that the optimal elevation to balance the
risks for both of these issues is unknown. They recommend
that until more evidence is available, caregivers should make
HOB elevation decisions in the context of the patient’s overall
condition. They recommend HOB elevation of 45 for patients
receiving EN who require mechanical ventilation or are heav-
ily sedated, but lowering the head to 30 might be done peri-
odically for patient comfort. They also stated that for critically
ill at less risk for aspiration (eg, non–mechanically ventilated
patients), it is recommended to maintain HOB at 30 and take
pressure-relieving measures.
Questions 6.5 and 6.6. Is elevated HOB required for
patients without significant aspiration risk? Are
there modes of ventilator support that can increase
the risk of aspiration (eg, high-volume flows, BIPAP,
APRV)?
Practice Recommendations
1. Maintain elevation of HOB at 30 or more for gastric
feeding. However, pump feeding interruption for short
periods of time to lower the HOB may not be necessary
or recommended unless contraindicated.
2. Consider carefully the indication for EN in the patient
receiving high-flow modes of ventilation, especially if
that patient is concomitantly receiving any sedation.
Rationale
When evaluating the research related to EN and aspiration risk,
it is important to note that much of this research has been con-
ducted in patients with critical care status, a factor that may
already increase aspiration risk. However, non–critically ill
patients may also be at risk for aspiration related to EN.
Patients requiring EN may not be able to protect their air-
way due to difficulty swallowing or other reasons, and aspira-
tion from oropharyngeal secretions may occur more readily in
the supine position. Patients in the supine position may be at
greater risk of aspiration due to gastric reflux than those whose
heads are elevated either in a bed or chair, while stopping a
slow-drip feeding for a brief period to reposition the patient in
bed may not be necessary and may even be counterproductive.
Assessment of the patient’s abdominal and bowel status to
check adequate gastrointestinal motility is an ongoing priority
in caring for the patient receiving EN. Returning the patient’s
HOB quickly to at least 30 is imperative.
34
High-flow ventilators and bag-valve-mask ventilations
increase likelihood of aspiration. However, these therapies are
essential in some situations because irreversible hypoxic brain
injury trumps the risk of potential aspiration. High-flow vol-
umes by noninvasive ventilation (NIV), noninvasive positive
pressure ventilation (NIPPV), or other means can increase the
risk of aspiration, and the risk is further increased in the sedated
patient.
16
EN is not always indicated in patients on high-flow
volume NIV as some patients have learned to eat with high-
flow volume NIV without incidence of pneumonia, including
patients with neuromuscular diseases such as amyotrophic lat-
eral sclerosis. Guidance from a speech and language patholo-
gist may help determine risk of aspiration, although eating may
be a quality-of-life issue for the patient who exercises self-
determination and elects to eat and drink while aware of the
risk of aspiration.
Meeting EN volume targets for patients with gastrostomy
tubes who are receiving respiratory therapies, especially with
high-pressure settings, is challenging. Patients who are receiv-
ing high-pressure respiratory support via NIV may experience
gastric insufflation. A patient with normal muscular function
may belch (eructate) to relieve the abdominal distention and
then be able to eat or take EN. However, a patient with a weak
diaphragm may be unable to belch and may experience gastric
bloating and fullness due to aerophagia. This phenomenon
happens when pressures to support respiration and the work of
breathing force air into the stomach. Early satiety and gastric
bloating may cause the patient to be unable to meet EN goals
due to feeling sated, sometimes despite feeling hungry. Venting
the gastric tube may relieve this condition and increase feeding
tolerance toward goals. Some medical centers have developed
aerodigestive clinics devoted to serving this client base. When
aggressive manual venting (eg, via open syringe) is not ade-
quate, a gastric decompression valve bag may provide addi-
tional relief and allow feeding toward volume goals.
35

Boullata et al 65
Carron and colleagues
36
reviewed optimal head position
and use of a nasogastric tube to ameliorate gastric distension,
although this review was unrelated to EN use. They detail the
sequelae whereby gastric distention compresses the lungs and
decreases compliance, which in turn demands higher airway
ventilation pressure. They suggest that airway pressures higher
than 20–25 cm H
2
O should be avoided. Moreover, considering
recent evidence of the efficacy of high-pressure NIV in severe
chronic hypercapnic COPD, this therapy should be carried out
in an almost sitting position approximately half an hour after a
meal or EN and with routine gastric decompression care.
37,38
Question 6.7. What factors determine the best duration
or rate of the feeding to improve the likelihood that
the full prescribed dose is received?
Practice Recommendations
1. Minimize interruptions to EN as much as possible to
help ensure optimal nutrition delivery.
2. Evaluate brief “NPO” status (eg, for procedures) for
need and minimize those interruptions as much as
possible. For example, the amount of time that a jejunal
feeding must be stopped for a procedure may be
different from the duration required for gastric feeding.
3. Accommodate interruptions to feeding delivery when
they are anticipated, and plan the feeding schedule to
maximize delivery of the daily feeding volume. A
volume-based feeding protocol may provide the nurse
with latitude in modifying EN administration to meet
the patient’s goal safely.
4. Consider patient condition factors and tolerance,
lifestyle, goals and convenience, and placement of the
distal end of the tube in formulating the feeding
regimen to meet patient nutrition and fluid needs.
Rationale
Various scheduling techniques for EN may be used in clinical
practice. Volume-based feeding protocols have been recom-
mended to ensure that patients receive adequate nutrition in a
given 24-hour period. In a pilot study, Heyland et al
9
demon-
strated improvement in nutrition delivery using volume-
based enteral feedings or the delivery of a daily feeding
volume target over a 24-hour period that prompts makeup of
missed feeding within set guidelines. McClave et al
39
evalu-
ated a volume-based feeding (VBF) protocol designed to
adjust for delivery interruptions in a prospective randomized
controlled trial compared to rate-based feeding (RBF) in
which the physician determined a constant hourly rate. On
days where feeding was interrupted, VBF patients received a
mean of 76.6% of goal calories vs the RBF group, which
received a mean of 61% of goal calories (P = .001); further-
more, VBF was not associated with vomiting, regurgitation,
or feeding intolerance. These investigators concluded that
VBF is safe and improves EN delivery compared to RBF.
In a prospective controlled trial where 164 critically ill
patients were randomly assigned to intermittent feeding (one-
sixth of the feeding goal was administered every 4 hours) vs
continuous feeding, both groups reached the feeding goal by
day 7, but the participants in the intermittently fed group
reached the goal faster and had a higher probability of being at
goal than those fed continuously.
40
Lichtenberg et al
41
found
that 158 patients scheduled for a 20-hour rate to compensate
for interruptions had a significantly reduced caloric deficit
(and a higher level of overfeeding) compared to 110 patients
fed for a 24-hour rate. Van den Broek and colleagues
42
observed
that administered feeding amounts were significantly lower
than prescribed in a 4-month study of 55 patients who received
continuous pump feeding, portion drip, or combined feeding
schedules. A mean energy deficit 1089 kJ/d (range, –7955 to
+795 kJ/d) was noted largely due to interruptions for proce-
dures. The delivered feeding was in goal range only in critical
care. They suggest adapting EN schedules to accommodate
periods when patients are off feedings as well as the use of
formulations with higher energy density.
Outcomes of these EN administration protocols may be dif-
ficult to demonstrate. de Araujo et al
43
studied 41 critically ill
patients who received continuous vs intermittent (per pump)
feeding and found no statistically significant difference in
terms of calories received per day, bowel distention, or emesis
for patients who had 6 hours off at night vs those fed for 24
hours per day. It has been suggested that feedings held for a
6-hour period might result in reduced gastric microbial growth
due to increased gastric acidity during the off period.
44
Patient convenience, lifestyle, and preferences are factors to
consider when creating the EN schedule, especially when EN
is likely to continue postdischarge. A 24-hour feeding schedule
is seldom needed, and periods without being connected to
feeding may enhance patient lifestyle. It may therefore be
advisable to individually assess the feeding schedule of each
patient, including those in long-term care settings.
Although jejunal feeding may be better tolerated as periodic
continuous feeding (eg, nocturnal feeding), the delivery sched-
ule options are limited compared to gastric feeding. Nocturnal
feeding may be used to encourage daytime oral intake; how-
ever, the patient’s appetite may still be dampened, and it may
be challenging to determine the adequacy of meals and modify
the EN volume accordingly. If oral intake is encouraged and a
gastric tube is being used, postmeal gravity bolus feeding can
be infused immediately after each meal to promote the patient’s
appetite for the next meal, and the amount of feeding can be
adjusted according to the adequacy of intake of each meal (eg,
use half of the EN volume after half of the meal is eaten).
When oral intake is discouraged (eg, because of marked dys-
phagia) but a patient is in an environment involving food, EN
can be administered prior to encounters with people eating to
dampen the patient’s appetite and reduce the desire to eat.

66 Journal of Parenteral and Enteral Nutrition 41(1)
When continuation of EN into the home setting is anticipated,
clinicians can implement the home schedule (such as gravity
bolus meal-like feedings) in the acute care setting before dis-
charge. This approach allows the acute care team to not only
work toward the feeding goal and assess patient tolerance but
also provide the patient or family as much assistance and train-
ing as possible before discharge.
Question 6.8. What practices maintain safety throughout
EN administration in regard to pump issues?
Practice Recommendations
1. Purchase best-performing pumps and follow
manufacturer recommendations for pump use and
maintenance.
2. Ensure that institutional biomedical engineering
departments periodically test, according to manufacturer
recommendations, whether pumps continue to meet the
accuracy rates and whether alarms function.
3. Consider a volume-based ordering system as opposed
to a rate-based delivery when appropriate to optimize
delivery of the total volume in a set time period.
4. Compare time of container initiation with completion
of infusion of container in terms of expected delivery
amounts as a double-check of accuracy of delivered
volume.
5. Zero the volume delivery amount on the feeding pump
at the beginning of a time period, such as usual intake
and output assessment period. This can serve as a check
of amount delivered, especially when that volume is the
same as the expected delivery volume. When the
volume delivered varies from expectations, additional
investigation regarding the variance is in order.
6. Use lightweight, portable, user-friendly, and accurate
pumps. For patients who may require continued pump
use in the home setting, consider the simplicity of use
and reliability of the pump. If possible, begin use of the
pump to be used in the home care setting before the
patient is discharged from acute care.
Rationale
Enteral feeding pumps are used to ensure accurate, consistent
feeding delivery with an alarm designed to signal interruption or
alteration to this delivery. Patients and caregivers who rely on
and are responsible to account for this consistent delivery expect
that an alarm will sound for any deviation from what is pre-
scribed in terms of delivery and that the volume-delivered fea-
ture represents actual volume delivered in a specific time period.
However, pumps have been shown to deliver rates and volumes
that vary from the prescribed settings.
45
Accuracy in delivery is
important for all who rely on enteral feeding pumps because
even small variances over time can have a significant impact on
the patient’s nutrition status. Particularly in vulnerable neonates
and young children, small differences in the rate and volume of
feeding can lead to major consequences.
White and King
46
discuss 4 areas for safety regarding the
use of enteral feeding pumps: (1) the consistent and accurate
delivery of formula, (2) the minimization of errors regarding
tube misconnection, (3) the impact of feed delivery itself, and
(4) the potentially toxic chemical composition of the casing
used in pump manufacture, although sets free of di(2-ethyl-
hexyl)phthalate (DEHP) are now marketed. They assert that
accuracy, safety, and consistency are important for patient con-
fidence and acceptance of feeding pumps.
The potential unreliability of pumps can be a source of
stress not only for staff and caregivers but also for patients,
including those in home settings, who may be concerned when
fluid remains in delivery containers at the end of a programmed
pump delivery period or, to the contrary, if feeding infuses
more quickly than expected. In 1 study of home EN in 34 pedi-
atric patients with inherited metabolic disorders, 75% of fami-
lies of children surveyed reported sleep disturbances related to
alarms, and 50% of home patients experienced faulty pumps
that affected accuracy and, in 1 critical incident, led to under-
feeding.
47
These authors published the review of enteral
pumps, suggesting that formula delivery is accurate to within
10% of what is programmed. Some pediatric and adult sys-
tems report adhering to deviance rates of only 5%.
46
Pump inaccuracy has been identified as a primary contribut-
ing factor in both underdelivery and overdelivery of feedings.
48
Tepaske et al
45
looked at 13 commercially available pumps
tested in a laboratory setting in 12 sessions with different tubes
and formulas. Formula delivery differed from preset to actual
delivery over a 24-hour period, with deficits ranging from
0.5%–13.5%, and differences of +66 mL to –271 mL per 24
hours. Decreased accuracy was attributed to the feeding pump
vs formula viscosity or resistance in delivery; however, only 1
pump of each type was tested in this study, and EADs varied
between 6 and 16 Fr in diameter. Spronk et al,
49
who tested 14
feeding pumps (6 Kangaroo 324 pumps and 8 Kangaroo 224
pumps), noted that discrepancies of up to 24 mL/h below the
preset volume occurred despite frequent calibrations by techni-
cal service using weight volume analysis. They discuss that dif-
ferences in delivered volumes could be due to viscosities of
formula or bending or twisting as the patient moves. They rec-
ommend monitoring pump function in various settings and con-
ditions, suggesting that technical service, age, and depreciation
of pumps influence their accuracy. For one brand of enteral
feeding pump, a 2011 report was issued to warn that users who
incorrectly pressed a certain key sequence might conclude that
an inoperable pump was infusing and consequently be at risk of
hypoglycemia due to lack of feeding.
50
Additionally, incorrect
key presses may cause a particular type of pump to appear to be
infusing even though an occlusion exists.
50
Older reports of
inaccuracies exist from 2003 and prior, but these findings may
not be generalizable to newer pumps.

Boullata et al 67
Manufacturers establish accuracy rates for their specific
pumps and generally fall within the accuracy rates as described
above.
46
Low-flow rates combined with high-dose settings
may exceed the life of the disposable set and should be replaced
every 24 hours to maintain delivery accuracy, allow proper air
and occlusion sensing, and prevent growth of bacteria.
Therefore, avoid programming a rate and dose combination
that exceeds a 24-hour feeding regimen. Pumps should be used
exclusively for enteral formulas or human milk and not inter-
changeably for medications and EN. When using HBM in
infants, syringe pumps are used to minimize the loss of HBM
in a feeding bag.
Question 6.9. Can the EN feeding system be a source for
contamination and infection and how can
contamination in the EN feeding system be best
prevented?
Practice Recommendations
1. Use a closed EN delivery systems when possible.
2. Follow the manufacturer’s recommendations for
duration of infusion through an intact delivery device
(container and administration set).
3. Do not reuse the enteral delivery device for open or
closed systems (container and administration set in
excess of what is recommended by the manufacturer).
4. If open systems are used, follow recommended hang
times and avoid topping off remaining formula, which
may result in a continuous culture for exponential
microbial growth.
a. Limit infusion time for open EN feeding systems
to 4–8 hours maximum (12 hours in the home
setting).
b. Limit infusion time for a reconstituted powder
product or modular to 4 hours maximum.
c. Change the delivery device (container and
administration set) according to the manufacturer’s
recommendations for open systems.
5. Be aware that the addition of modular units to an open
feeding system may result in an unacceptable risk of
contamination in hyperthermal environments.
6. To limit the risk of microbial growth and biofilm
formation, avoid unnecessary additions to the EN
administration set. If additional equipment, such as
3-way stopcocks, are used, follow manufacturer
recommendations or facility protocol for change and
cleaning practices.
7. Establish and follow protocols for preparation,
handling, and storage of commercial and handmade
EN.
a. Educate those who prepare and administer EN
about hand hygiene (a critical point) and safe
handling of EN preparation and administration;
extend education to patients and family members/
care givers who will continue this practice into
the home setting.
b. Use effective hand hygiene in all aspects of EN
preparation and administration. When gloves are
used, they must be clean gloves, not having been
involved in other nonrelated tasks. The importance
of hand washing in minimizing transference of
microbial growth and preventing hospital-
acquired infections cannot be overstressed.
c. Give preference to selecting systems that require
minimal handling.
d. Use a clean work surface for EN preparation.
e. Use equipment dedicated for EN use only.
f. Store EN formula according to the manufacturer’s
instructions. Store prepared or opened ready-to-
feed solutions in an appropriate refrigerator,
discarding any used solutions within 24 hours of
preparation or opening.
8. Periodically survey and regularly monitor adherence
to the above-listed protocols. Document findings and
take appropriate actions if protocols are not followed.
9. Reduce potential for touch contamination of
EN-related equipment as well as risk of exposure to
body fluids by reducing interruptions to the system,
providing a clean work surface (eg, small clean towel
under tube/administration connection) and when
interruptions are necessary, and using only washed
hands and gloves.
10. Keep all equipment, including syringes and containers
for flush and medication administration, as clean and
dry as possible. Store clean equipment away from
potential sources of contamination.
11. Consider whether microbial growth related to EN
might be implicated as part of the diagnosis when
patients have adverse conditions such as diarrhea.
Rationale
Although microbial growth has been associated with EN in a
variety of studies and in a variety of ways, contamination
related to EN is an often overlooked source of bacterial infec-
tion.
1,51
In discussing microbial growth, questions arise such as
which types and what amount of microorganisms are harmful,
what are the associated adverse effects of harmful microbial
growth, and what areas related to EN are most strongly corre-
lated with harmful microorganisms.
52
Patients who require EN
may be immunocompromised, at least until their nutrition sta-
tus is improved, and they rely on healthcare professionals to
minimize risk related to EN delivery.
Hospital-prepared EN poses the risk for foodborne illness
or nosocomial infection.
38,53,54
Blenders used in reconstituting
formulas have been cited as a primary source of contamina-
tion.
55
Diluting formula hung for a period of time is no longer

68 Journal of Parenteral and Enteral Nutrition 41(1)
recommended because additions to the EN system increase
risk of microbial growth.
Water that is hung as a separate infusion to the EN delivery
device may also serve as a source for exponential microbial
growth, especially when the water is hung for extended periods
(eg, >8–24 hours); however, reporting of well-designed research
in this area is lacking.
In a prospective, descriptive study, cultures were taken
from 30 pediatric patients every 4 hours as they were adminis-
tered continuous feeding of decanted formula over a minimum
hang time of 12 hours with formula added per “current prac-
tice.” Out of 111 usable cultures, 100 had no growth, 6 had
growth below the FDA threshold for contamination, and 5 cul-
tures in 2 patients grew coliforms with no evidence of bacterial
gastroenteritis over the 48-hour data collection period.
56
In this
study, decanted formula used for pediatric patients had a lower
growth rate over a 12-hour period than anticipated when rec-
ommended handling procedures were followed.
Perry and colleagues
57
compared closed EN systems with
open systems and open systems with modular additives in a criti-
cal care burn unit. No microbial growth was found in closed and
open systems in the thermoneutral and hyperthermal critical care,
nonpatient environment, although humidity was not reported.
Microbial growth was noted in both temperature environments in
the open system with modular additives. Significant growth in
the open system with modular additives was noted in the hyper-
thermal environment, where 30% of samples exceeded FDA
standards by 4 hours and CFUs were too numerous to count by 8
hours. These investigators concluded that the addition of modular
units to an open feeding system may result in an unacceptable
risk of contamination in hyperthermal environments.
A wide variety of organisms was recovered from neonatal
feeding tubes in studies by Juma and Forsythe
58
and Hurrell
et al.
59
In Juma and Forsythe’s study, some of the organisms
were encoded for antibiotic resistance.
58
Hurrell and colleagues
reported that a multitude of organisms, including antibiotic-
resistant ones, was identified in 129 feeding tubes collected
from 2 neonatal intensive care units (NICUs), and Klebsiella
pneumoniae and Serratia marcescens caused infections in the
2 NICUs.
59
The significance of biofilm formation in enteral
feeding tubes, which constitutes a risk factor for susceptible
neonates, is highlighted in another report by this group of
investigators.
60
Biofilm growth on 3-way stopcock valves used
within the feeding delivery system can cause nosocomial
infections; Pseudomonas aeruginosa was found to develop a
bacterial biofilm in these valves within 3 days.
61
These valves
may be used with no routine change time or care practices and
may be exposed to many interruptions and manipulations.
System design has been suggested to play an important role
in reducing bacterial contamination. Retrograde spread of the
patient’s own flora has been identified as a source of contami-
nation in EN administration sets, and system design improve-
ments (such as recessed spikes on administration sets) have
been recommended to reduce potential touch contamination.
62
Mathus-Vliegen et al
51
reported that the large amount of
potentially pathogenic bacteria found in delivery sets was
likely related to the endogenous vs exogenous route, poten-
tially due to retrograde microbial growth.
In a study of EN-related equipment, clean, dry feeding
equipment had less microbial growth than feeding equipment
that retained moisture, feeding formula, and other media for
microbial growth. Syringes stored for up to 5 days in a clean,
dry fashion as 2 pieces (ie, piston being removed from the bar-
rel of the syringe prior to storage) had less microbial growth
than more newly obtained syringes (eg, 12 hours) that housed
moisture where cultures exceeded standards for both type and
amount of microbial growth. Also noted, feeding tubing
administration caps taped upright to IV poles had significantly
more adverse microbial growth cultured from them than caps
that were stored in a manner to prevent moisture retention.
52
Ho and colleagues
63
found a strong correlation between cul-
tures taken from staff hands and contamination of tube hubs,
enteral feeding, and nasopharynx and gastric fluid, and the
investigators noted a significant reduction in contamination in
the group that received an infection control program (ICP).
Hand contamination with methicillin-resistant Staphylococcus
aureus (MRSA) was highly correlated with contamination of the
EN system, and these authors recommend ICPs in long-term
care settings. The effect of touch contamination has been dem-
onstrated in syringes,
64
and healthcare professionals must take
measures to avoid the transfer of microbial growth from hands to
patient care items and areas, such as the inner aspect of a feeding
tube. The importance of appropriate hand hygiene and clean
glove use as indicated cannot be overstressed. Additionally, a
clean surface (eg, a clean small towel under tubing prior to dis-
connections or manipulation) may reduce inadvertent touch con-
tamination from less clean areas. Changing delivery systems at
once is less risky than topping off the volume of formula.
Reuse of feeding bags for the home setting is sometimes
considered a cost-saving measure. Oie and Kamiya
65
found that
washing feeding bags with water and then 0.1% sodium hypo-
chlorite (ie, bleach) solution significantly reduced microbial
growth (P < .01) compared with washing with water alone.
Rinsing of continuous EN sets used for 24 hours with tap water
was not determined to decrease contamination when cultured at
8 and 16 hours in a 2-group comparison (rinse vs nonrinse).
66
Williams and colleagues
67
conducted a randomized controlled
trial and concluded that aspirating GRVs less frequently in critical
care was not correlated with increased patient risk of complica-
tions from EN but could potentially reduce the risk of contamina-
tion of the feeding circuit and the risk of exposure to body fluid. In
another study, Williams et al
16
identified other strategies to reduce
interruptions to enteral feeding that might increase risks of con-
tamination and negatively affect nutrition outcomes.
Adverse events related to microbial growth in EN have
been addressed, but additional research in this area may
prove to be of benefit. Clostridium difficile and associated
diarrhea in hospitalized tube-fed patients have been corre-
lated with EN, especially in those receiving postpyloric
feeding.
68,69
With the steady increase in this very serious

Boullata et al 69
malady, every potential correlation must be considered,
including medications, underlying disease, and prior status,
but bacterial contamination must also be considered.
70
There
are many potential causes of frequent and/or loose stools,
including medications, underlying disease, and prior status,
but bacterial contamination must be considered.
70
In an
observational, retrospective study of EN use in 175 hospital-
ized poststroke patients compared 24-hour hang time vs
72- or 96-hour hang time, the 24-hour hang time was inde-
pendently associated with a lower risk of diarrhea and longer
diarrhea-free survival.
71
Jack et al
72
reported a 78% inci-
dence of diarrhea in 55 patients using EN, and the frequency
increased with longer periods of enteral feeding. They rec-
ommended that organizations use a diarrhea risk manage-
ment algorithm. Hurt et al
73
suggested that incorporation of
EN as a base strategy for stress ulcer prophylaxis to reduce
the need for acid-suppressive therapy may reduce C difficile
pseudomembranous colitis. Others have recommended
allowing stopping EN for periods of time (eg, 6-hour break)
to allow gastric pH to return to its more normal acidic pH to
help reduce gastric microbial growth.
44
Healthcare organizations that follow national standards
practice recommendations (eg, Hazard Analysis and Critical
Control Point [HACCP] and National Institute for Health and
Clinical Excellence [NICE] 2012) in training and monitoring
staff who work with EN can reduce and contain microbial
growth.
74,75
For example, Oliveira et al
55
reported that a hospital
reduced bacterial count from 10
5
CFU/mL to 10
1
CFU/mL by
following HACCP guidelines for preparation, storage, and
delivery of enteral feeds and using a flowchart and monitoring
critical control points defined using a decision tree based on
HACCP guidelines. If using a threshold of 10
5
CFU/mL, then
EN delivery sets should be used within 24 hours.
66
See Figure 9
for hang times for EN and Figure 10 for an overview of poten-
tial contamination points in EN.
Question 6.10. Under what circumstances (if any) should
EN be held to improve patient safety (prior to
transportation, prior to procedures, surgery, or
extubation)?
Practice Recommendations
1. Avoid interruptions or holding EN for routine
interventions, including endotracheal extubation and
procedures where short periods of HOB lowering are
needed.
a. Perform a thorough assessment for oropharyngeal
secretion retention and potential for reflux of
gastric fluid by a qualified professional.
b. Disconnection of EN equipment not only
decreases nutrition delivery and increases
potential microbial growth of related equipment
but also increases the risk for tubing misconnection.
2. Consider risk vs benefit regarding disconnection of EN
on an individual basis as it reduces needed nutrient
delivery and may increase safety risk.
3. Follow the American Society of Anesthesiologists
preoperative fasting recommendations
76
:
a. Human milk—4 hours
b. Infant formula—6 hours
c. Nonhuman milk—6 hours
Figure 9. Hang times for enteral nutrition. HBM, human breast milk; IC, immunocompromised; PDM, pasteurized donor milk.

70 Journal of Parenteral and Enteral Nutrition 41(1)
Rationale
Safety can be built into all aspects of patient care, and ownership
for safety integration must be an expectation of all healthcare
professionals. When EN is held for tests and procedures, patients
are deprived of nutrition and fluid unless lost volume is effec-
tively made up during the other hours of the 24-hour period.
Peev et al
77
compared avoidable and unavoidable interruptions
in EN and equated interruptions in EN delivery to undesirable
outcomes such as underfeeding and prolonged length of hospi-
talization. Withholding feeding can be done as necessary, but
decisions based solely on tradition are not advisable. Instead,
clinicians are encouraged to use evidence and critical thinking to
decide whether to interrupt feedings. Williams and colleagues
16
have reviewed means to reduce avoidable interruptions.
Transporting patients between departments, areas, facilities, or
care settings increases the potential for disconnection and miscon-
nection of the enteral feeding system, delay of feeding resump-
tion, and potential tube clogging, as well as deviation from usual
preventive practices, such as maintaining HOB elevation.
Intrahospital transportation has been identified as a risk factor for
pneumonia. In a cohort-matched design study of critically ill ven-
tilated patients, 118 patients were transported (primarily for radio-
logic procedures) and 118 were not. Of those who were transported,
26% developed ventilator-associated pneumonia (VAP), as
opposed to 10% of those who were not transported.
78
Three inde-
pendent risk factors for VAP were identified in this study: the need
for reintubation, EN, and intrahospital transport. It was not clear
whether alteration in HOB positioning was a factor in these
outcomes. During transport, appropriate hand-off between quali-
fied personnel is essential. Documentation of line tracing and
ready access provide resources if concerns or questions arise.
Depending on the context, turning continuous EN off for
lowering the HOB for a brief time may be unnecessary and even
counterproductive in terms of reduced feeding volume, risk of
forgetting to turn the feeding back on, and increased potential for
tube clogging. If the HOB must be lowered, it should be quickly
reelevated to 30°, or preferably 45°, unless contraindicated.
28,34
Another possible option is to reposition the patient in reverse
Trendelenburg while feedings infuse. The patient clinical condi-
tion may be a more influential risk factor for reflux and aspira-
tion than the small per-minute volume of feeding delivery.
Oropharyngeal suctioning and assessment of patient condition,
including abdominal assessment, may be more helpful in tem-
pering aspiration risk than stopping small-volume feeding infu-
sion for a short period for lowering the HOB.
The standard practice of NPO after midnight prior to proce-
dures and surgery has been challenged and warrants patient-
specific consideration regarding its appropriateness and risks
and benefits.
79
For example, jejunal feeding may not need to be
held for the same time period as gastric feeding, especially
when gastric decompression may be an option prior to a proce-
dure. In a study by Moncure and colleagues,
80
46 patients with
jejunal tube feeding that infused until they were transported to
the operating room were compared to 36 patients who had jeju-
nal feeding held for 8 hours prior to surgery. No aspiration was
noted in either group, and the investigators concluded that jeju-
nal feeding may safely continue until the time of surgery.
Figure 10. Contamination points in formula preparation. EAD, enteral access device; EN, enteral nutrition; HBM, human breast milk;
PDM, pasteurized donor milk.

Boullata et al 71
In a prospective, observational cohort study, critically ill,
mechanically ventilated patients were fed via gastric tube until
45 minutes prior to selected operative and nonoperative proce-
dures or via duodenal tube until the procedure started. Pousman
and colleagues
81
found a trend in the intervention group toward
increased nutrition administration and faster attainment of target
goals, with no statistically significant difference between the usual
practice group and the patients with the reduced fasting protocol.
The American Society of Anesthesiologists have published
practice guidelines for preoperative fasting timeframes for elec-
tive procedures. These include discontinuing various liquids
prior to an elective surgical procedure. Those liquids pertinent
to the patient receiving EN include human milk, infant formula,
and nonhuman milk. A 2-hour fasting time period for those
receiving human milk is recommended, a 4-hour time period is
recommended for infant formula, and a 6-hour time fasting
period is recommended for those receiving nonhuman milk.
76
The practice of holding EN for patient conditions also war-
rants critical appraisal. For example, McClave and Chang
82
have
concluded that “evidence of gastrointestinal bleeding is not an
automatic contraindication” to EN; rather, EN may protect the
gut mucosa and further reduce bleeding, increase the risk for
rebleeding, or “serve as a moot point with no relation to further
bleeding.” They discuss reasons to consider continuing or hold-
ing feeding for a period of time, depending on etiology of the
bleeding. Other decisions about interrupting EN, such as whether
to hold feeding for a period prior to endotracheal extubation or
for medication administration, will also depend on the specific
situation and the best evidence available to the clinician.
Question 6.11. What is the most accurate method to
measure the amount of formula infused (ie, recorded
I/O, marking the bottle or bag)? Who is responsible
for monitoring whether the amount recorded was
actually infused?
Practice Recommendations
1. Do not rely on pump rate and volume settings alone to
determining the amount of feeding infused. Calculate
the hourly rate multiplied by the hours infused, allotting
for any downtime and use other methods to double
check and ensure accuracy of volume infused. Compare
that volume to the pump history of volume infused for
an accurate measure of intake.
2. Document the volume of EN and other fluid
administered and investigate when suboptimal nutrition
and fluid seems to have been delivered. Serve as patient
advocates to promote best nutrition and fluid delivery.
3. Monitor nutrition and fluid trends, including any gaps
in delivery, and pursue methods to enhance delivery as
indicated.
4. Implement methods to ensure that adequate nutrition is
being administered for patients who continue EN after
they transition from acute care to another setting.
5. Tailor ordering methods to help ensure that accurate
nutrition volumes are delivered:
a. Consider volume-based feeding schedules where
a specific volume is to be infused in a 24- hour
period.
b. Use an easily measurable volume, such as one or
two 1-liter containers/d or 2 cartons (cups) of
feeding per EN “meal,” in orders for EN in the
home care setting.
6. Institute systems to embed accountability and oversight
for accurate delivery of nutrition intake, including
methods of ordering and documenting actual intake.
Have policies and procedures to determine whether
systems are suboptimal or break down, and use system
improvement methods to address problems.
7. Encourage use of electronic connectivity between
enteral pump and the intake portion of the EHR to
document EN volume infused.
Rationale
Many stakeholders are involved in ensuring that adequate feed-
ing volumes are infused, including the patient/family, direct
care staff, and those who oversee specific aspects or the overall
management of the patient course, from recovery to healing and
maintenance. Daily care staff are responsible to account for EN
infusion volume over a specific period. If the infusion rate is
multiplied by the number of hours infused, there is a risk that
periods when feeding was held may be inadvertently omitted
from the intake record. Feeding pump infusion volume may
also be an unreliable measure. Volume-based ordering has been
recommended over rate-based ordering for more accurate EN
delivery.
39,48,83
Sometimes, staff or patients themselves question
why 100 mL of EN remains after an overnight infusion when
the total volume should have infused. However, when the less-
than-optimal infusion volume is not noticed, nutrition deficits
can accrue. Professionals who oversee the broad aspects of EN
delivery volume use records of daily feeding volumes to assess
the overall EN delivery trend and its effects. They may be
responsible for establishing and updating the nutrition plan
based on trends and outcomes. Delivery and calculation of EN
formula may be more accurate when volumes can be ordered in
specific amounts, such as 2 cartons/cans/cups of feeding 3
times per day or one 1000-mL container per night. Similarly, if
water intake is ordered in specific amounts and accountability
for it is built into the EHR, such as via the medication adminis-
tration record, delivery may be more reliable and accurate.
Also, when water is described in terms of household measure-
ments, such as a cup of water, the patient, family, and staff
might more easily equate feeding to meals.
Enteral feeding pump inaccuracy contributes to the dis-
crepancy between ordered and delivered formula volume.
Feeding pumps may either overdeliver or underdeliver pre-
scribed volume within the prescribed timeframe.
84–86
Deficits
of 0.5%–21% have been observed.
84,85
The set rate on the

72 Journal of Parenteral and Enteral Nutrition 41(1)
pump does not always correlate with the amount of formula
delivered, and this discrepancy may be responsible for up to
81% of cases where the patient does not receive the prescribed
amount of formula.
76
Advances in enteral feeding pump tech-
nology may improve accuracy.
Double-checks and assessments for accuracy of delivered
amounts such as comparing formula amount and time hung
with amount remaining at the end of a time period compared to
expected delivered amount can help detect inaccuracies of EN
delivery.
Topics for Future Research
Comparison of gastric vs small bowel feedings on
clinical outcomes in patients requiring prone positioning
The advantages and disadvantages of holding enteral
feedings for surgical procedures and for what duration
prior to the procedure
Incidence of overt or microaspiration in patients fed via
the bolus method
Jejunal feeding transition from continuous to
intermittent or bolus method for patient convenience
Feasibility of transferring enteral volume data directly
from enteral feeding pump to the EHR
References
1. Bankhead R, Boullata J, Brantley S, et al. Enteral nutrition practice recom-
mendations. JPEN J Parenter Enteral Nutr. 2009;33:122-167.
2. American Association of Critical-Care Nurses. AACN Practice Alerts:
prevention of aspiration. Crit Care Nurs. 2016;36(1):e20-e24.
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provi-
sion and assessment of nutrition support therapy in the adult critically ill
patient: Society of Critical Care Medicine (SCCM) and American Society
for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter
Enteral Nutr. 2016;40(2):159-211.
4. Mackenzie SL, Zygun DA, Whitmore BL, Doig CJ, Hameed SY.
Implementation of a nutrition support protocol increases the proportion of
mechanically ventilated patients reaching enteral nutrition targets in the
adult intensive care unit. JPEN J Parenter Enteral Nutr. 2005;29(2):74-80.
5. McCarthy MS, Martindale RG. What’s on the menu? Delivering evi-
dence-based nutritional therapy. Nursing. 2015;45(8):36-44.
6. Ventura A, Cordeiro M, Waitzberg DL. Enteral nutrition protocols for
critically ill patients: are they necessary? JPEN J Parenter Enteral Nutr.
2015;30(3):351-362.
7. Martin CM, Doig GS, Heyland DK, Morrison T, Sibbald WJ; Southwestern
Ontario Critic Care Research Network. Multicentre, cluster-randomized
clinical trial of algorithms for critical-care enteral and parenteral therapy
(ACCEPT). CMAJ. 2004;170(2):197-204.
8. Stewart ML. Nutrition support protocols and their influence on the deliv-
ery of enteral nutrition: a systematic review. Worldviews Evidence Based
Nurs. 2014;11(30):194-199.
9. Heyland DK, Cahill NE, Dhaliwal R, Sun X, Day AG, McClave SA. Impact of
enteral feeding protocols on enteral nutrition delivery: results of a multicenter
observational study. JPEN J Parenter Enteral Nutr. 2010;34(6):675-684.
10. Racco M. An enteral nutrition protocol to improve efficiency in achieving
nutritional goals. Crit Care Nurs. 2012;32(4):72-75.
11. Kenny DJ, Goodman P. Care of the patient with enteral tube feeding: an
evidence based practice protocol. Nurs Res. 2010;59(1):S22-S31.
12. Yantis MA, Velander R. Untangling enteral nutrition guidelines. Nursing.
2011;41(9):32-38.
13. Guenter P, Hicks RW, Simmons D, et al. Enteral feeding misconnec-
tions: a consortium position statement. Jt Comm J Qual Patient Saf.
2008;34:285-292.
14. Dhaliwal R, Cahill N, Lemieus M, Heyland DK. The Canadian critical care
nutrition guidelines I 2013: an update on current recommendations and
implementation strategies. JPEN J Parenter Enteral Nutr. 2014;29(1):29-43.
15. Lyerla F, LeRouge C, Cooke DA, Turpin D, Wilson L. A nursing clinical
decision support system and potential predictors of head-of-bed position for
patients receiving mechanical ventilation. Am J Crit Care. 2010;19(1):39-47.
16. Williams TA, Leslie GD, Leen T, Mills L, Dobb GJ. Reducing interrup-
tions to continuous enteral nutrition in the intensive care unit: a compara-
tive study. J Clin Nurs. 2013;22:2838-2848.
17. Clark JR. What’s in your policy manual? Air Med J. 2011;30(1):18-19.
18. The Joint Commission. Sentinel Event Alert #53: Managing risk during
transition to new ISO tubing connector standards. August 2014. https://
www.jointcommission.org/sea_issue_53/
. Accessed May 12, 2016.
19. Guenter P. Safe practices for enteral nutrition in critically ill patients. Crit
Care Nurs Clin North Am. 2010;22(2):197-208.
20. Halm MA, Armola R. Effect of oral care on bacterial colonization and
ventilator-associated pneumonia. Am J Crit Care. 2010;18:275-278.
21. American Association of Critical-Care Nurses. AACN Practice Alert: oral
care for patients at risk of ventilator-associated pneumonia. April 2010.
http://www.aacn.org/wd/practice/docs/practicealerts/oral-care-patients-
at-risk-vap.pdf?menu=aboutus
. Accessed May 18, 2016.
22. Metheny NA, Davis-Jackson J, Stewart BJ. Effectiveness of an aspiration
risk-reduction protocol. Nurs Res. 2010;59(1):18-25.
23. Metheny NA. Preventing respiratory complications of tube feedings: evi-
dence-based practice. Am J Crit Care. 2006;15(4):360-369.
24. Ellett ML, Beckstrand J, Flockier J, Perkins SM, Johnson CS. Predicting the
insertion distance for placing gastric tubes. Clin Nurs Res. 2005;14(1):11-27.
25. Ellett ML, Cohen MD, Perkins SM, Croffie JM, Lane KA, Austin JK.
Comparing methods of determining insertion length for placing gastric tubes
in children 1 month to 17 years of age. J Spec Pedia Nurs. 2012;17(1):19-32.
26. Chen Y, Want L, Chang Y, et al. Potential risk of malposition of nasogastric
tube using nose-ear-xiphoid measurement. PLoS One. 2014;9(2):e88046.
27. Task Force on Sudden Infant Death Syndrome, American Academy of
Pediatrics. SIDS and other sleep related infants deaths: expansion of
recommendations for a safe infant sleeping environment. Pediatrics.
2011;128:1030-1039.
28. Metheny NA, Stewart BJ, McClave SA. Relationship between feed-
ing tube site and respiratory outcomes. JPEN J Parenter Enteral Nutr.
2011;35(3):346-355.
29. Heyland DK, Drover JW, MacDonald S, Novak F, Lam M. Effect of
postpyloric feeding on gastroesophageal regurgitation and pulmonary
microaspiration: results of a randomized controlled trial. Crit Care Med.
2001;29(8):1495-1501.
30. Linn DD, Beckett, RD, Foellinger K. Administration of enteral nutrition to
adult patients in the prone position. Intensive Crit Care Nurs. 2015;31:38-43.
31. Fineman LD, LaBrecque M A, Mei-Chiung Shih, Martha AQ. Prone posi-
tion can be safely performed in critically ill infants and children. Pediatr
Crit Care Med. 2006;7(5):413-422.
32. National Pressure Ulcer Advisory Panel. European Pressure Ulcer advi-
sory Panel and Pan Pacific Pressure Ulcer Alliance. Prevention and
Treatment of Pressure Ulcers. Perth, Australia: Cambridge Media; 2014.
33. Schallom M, Dykeman B, Metheny N, Kirby J, Pierce J. Head-of-bed ele-
vation and early outcomes of gastric reflux, aspiration and pressure ulcers:
a feasibility study. Am J Crit Care. 2015;24(1):57-66.
34. Metheny N. Turning tube feeding off while repositioning patients in bed.
Crit Care Nurs. 2011;31(2):96-97.
35. Loh LE, Chan YH, Chan I. Noninvasive ventilation in children: a review.
J Pediatr (Rio J). 2007;83(2)(suppl):S91-S99.
36. Carron M, Freo U, BaHammam AS, et al. Complications of non-invasive
ventilation techniques: a comprehensive qualitative review of randomized
trials. Br J Anaesth. 2013;110(6):896-914.
37. Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med.
2001;163:540-577.

Boullata et al 73
38. Luria O, Reshef L, Barnea O. Analysis of non-invasive ventilation effects
on gastric inflation using a non-linear mathematical model. Resuscitation.
2006;71:358-364.
39. McClave SA, Saad MA, Esterle M, et al. Volume-based feeding in the
critically ill patient. JPEN J Parenter Enteral Nutr. 2015;39(6):707-712.
40. MacLeod JB, Lefton J, Houghton D, et al. Prospective randomized control
trial of intermittent versus continuous gastric feeds for critically ill trauma
patients. J Trauma Injury Infect Crit Care. 2007;63(1):57-61.
41. Lichtenberg K, Guay-Berry P, Pipitone A, Bondy A, Rotello L.
Compensatory increased enteral feeding goal rates: a way to achieve opti-
mal nutrition. Nutr Clin Pract. 2010;25(6):653-657.
42. Van den Broek PW, Rasmussen-Conrad EL, Naber AH, Wanten GJ. What
you think is not what they get: significant discrepancies between prescribed
and administered doses of tube feeding. Br J Nutr. 2009;101(1):68-71.
43. de Araujo VM, Gomes PC, Caporossi C. Enteral nutrition in critical
patients: should the administration be continuous or intermittent? Nutr
Hosp. 2014;29(30):563-567.
44. Stroud M, Duncan H, Nightingale J; British Society of Gastroenterology.
Guidelines for enteral feeding in adult hospital patients. Gut. 2003;52(suppl
7):Vii1-Vii12.
45. Tepaske R, Binnekade JM, Goedhart PT, Schultz MJ, Vroom MB,
Mathus-Vliegen EM. Clinically relevant differences in accuracy of
enteral nutrition feeding pump systems JPEN J Parenter Enteral Nutr.
2006;30(4):339-343.
46. White H, King L. Enteral feeding pumps: efficacy, safety, and patient
acceptability. Med Devices Evid Res. 2014;7:291-298.
47. Evans S, MacDonald A, Daly A, Hopkins V, Holden C. Home enteral
tube feeding in patients with inherited metabolic disorders: safety issues. J
Hum Nutr Diet. 2007;20(5):440-445.
48. Walker RN, Utech A, Velex ME, Schwartz K. Delivered volumes of enteral
nutrition exceed prescribed volumes. Nutr Clin Pract. 2014;29(5):662-666.
49. Spronk PE, Rommes JH, Kuiper MA. Structural underfeeding due to inac-
curate feeding pumps? JPEN J Parenter Enteral Nutr. 2007;31(2):154.
50. ECRI Institute. Hazard report: incorrect key presses may cause Nutricia
Flocare Infinity Series enteral feeding pumps to appear to be infusing even
though and occlusion exists. Health Devices. May 2011:170-171.
51. Mathus-Vliegen EA, Bredius MW, Binnekade JM. Analysis of bacterial
contamination in an enteral feeding system. JPEN J Parenter Enteral
Nutr. 2006;30(6):519-525.
52. McGinnis C. The Prevalence of Microbial Growth in Enteral Tube
Feeding in Two North Central Hospitals [master’s thesis]. Brookings:
South Dakota State University; 1995.
53. Baniardalan M, Sabzghabaee AM, Jalali M, Badri S. Bacterial safety of
commercial and handmade enteral feeds in an Iranian teaching hospital.
Int J Prev Med. 2014;5(5):604-610.
54. Jalali M, Sabzghabaee AM, Badri SS, Soltani HA, Maracy MR. Bacterial
contamination of hospital-prepared enteral tube feeding formulas in
Isfahan, Iran. J Res Med Sci. 2009;14(3):149-156.
55. Oliveira MR, Batista CR, Aidoo KE. Application of hazard analysis criti-
cal points system to enteral tube feeding in hospital. J Hum Nutr Diet.
2001;14(5):397-403.
56. Lyman B, Gebhards S, Hensley C, Roberts C, San Pablo W. Safety of
decanted enteral formula hung for 12 hours in a pediatric setting. Nutr Clin
Pract. 2011;26(4):451-456.
57. Perry J, Stankorb SM, Salgueiro M. Microbial contamination of enteral
feeding products in thermoneutral and hypothermal ICU environments.
Nutr Clin Pract. 2015;30(1):128-133.
58. Juma NA, Forsythe SJ. Microbial biofilm development on neonatal enteral
feeding tubes. Adv Exp Med Biol. 2015;830:113-121.
59. Hurrell E, Kucerova E, Loughlin M, et al. Neonatal enteral feeding tubes
as loci for colonization by members of the Enterobacteriaceae. BMC Infect
Dis. 2009;9:146.
60. Hurrell E, Kucerova E, Loughlin M, Caubilla-Barron J, Forsythe SJ.
Biofilm formation on enteral feeding tubes by Cronobacter sakazakii,
Salmonella serovars and other Enterobacteriaceae. Int J Food Microbiol.
2009;136(2):227-231.
61. Solseng T, Vinson H, Gibbs P, Greenwald B. Biofilm growth on the Lopez
enteral feeding valve cultured in enteral nutrition: potential implications
for medical-surgical patients, nursing care and research. Medsurg Nurs.
2009;18(4):225-233.
62. McKinlay J, Wildgoose A, Wood W, Gould IM, Anderton A. The effect
of system design on bacterial contamination of enteral tube feeds. J Hosp
Infect. 2001;47(2):138-142.
63. Ho SS, Tse MM, Boost MV. Effect of an infection control program on
bacterial contamination of enteral feed in nursing homes. J Hosp Infect.
2012;82(1):49-55.
64. Stucki C, Sautter A, Favet J, Bonnabry P. Microbial contamination of
syringes during preparation: the direct influence of environmental cleanli-
ness and risk manipulations on end-product quality. Am J Health System
Pharm. 2009;66:2032-2036.
65. Oie S, Kamiya A. Comparison of microbial contamination of enteral
feeding solution between repeated use of administration sets after wash-
ing with water and after washing followed by disinfection. J Hosp Infect.
2001;48(4):304-307.
66. Kohn CL. The relationship between enteral formula contamination and
length of enteral delivery set usage. JPEN J Parenter Enteral Nutr.
1991;15:567-571.
67. Williams TA, Leslie G, Mills L, et al. Frequency of aspirating gastric
tubes for patients receiving enteral nutrition in the ICU: a randomized
controlled trial. JPEN J Parenter Enteral Nutr. 2014;38(7):809-816.
68. Bliss DZ, Johnson S, Savik K, Clabots CR, Willard K, Gerding DN.
Acquisition of Clostridium difficile and Clostridium difficile–associated
diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med.
1998;129(12):1012-1019.
69. Bliss DZ, Johnson S, Savik K, Clabots CR, Gerding DN. Fecal incontinence
in hospitalized patients who are acutely ill. Nurs Res. 2000;49(2):101-108.
70. Chang SJ, Huang HH. Diarrhea in enterally fed patients: blame the diet?
Curr Opin Clin Nutr Metab Care. 2013;16(5):588-594.
71. Arevalo-Manso JJ, Martinez-Sanchez P, Juares-Martin B, et al. Preventing
diarrhea in enteral nutrition: the impact of the delivery set hang time. Int J
Clin Pract. 2015;69(8):900-908.
72. Jack L, Coyer F, Courtney M, Venkatesh B. Diarrhea risk factors in enter-
ally tube fed critically ill patients: a retrospective audit. Intensive Crit
Care Nurs. 2010;26(6):327-334.
73. Hurt RT, Frazier TH, McClave SA, et al. Stress prophylaxis in inten-
sive care unit patients and the role of enteral nutrition. JPEN J Parenter
Enteral Nutr. 2012;36(6):721-731.
74. Food and Drug Administration. Hazard Analysis Critical Control
Point (HACCP). August 14, 1997. http://www.fda.gov/Food/
GuidanceRegulation/HACCP/ucm2006801.htm
. Accessed May 18, 2016.
75. National Institute for Health and Clinical Excellence. Infection Control:
Prevention and Control of Healthcare-Associated Infections in Primary
and Community Care. London, UK: NICE; 2012. http://pathways.nice.
org.uk/pathways/prevention-and-control-of-healthcare-associated-
infections#path=view%3A/pathways/prevention-and-control-of-health-
care-associated-infections/enteral-feeding-prevention-and-control-
of-healthcare-associated-infections-in-primary-and-community-care.
xml&content=view-index
. Accessed August 8, 2015.
76. American Society of Anesthesiologists Committee on Standards and
Practice Parameters. Practice guidelines for preoperative fasting and
the use of pharmacologic agents to reduce the risk of pulmonary aspi-
ration: application to healthy patients undergoing elective procedures.
Anesthesiology. 2011;114:495-451.
77. Peev MP, Yea DD, Quraishi SA, et al. Causes and consequences of inter-
rupted enteral nutrition: a prospective observational study in critically ill
surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27.
78. Bercault N, Wolf M, Runge I, Fleury JC, Boulain T. Intrahospital transport
of critically ill ventilated patients: a risk factor for ventilator-associated pneu-
monia—a matched cohort study. Crit Care Med. 2005;33(11):2471-2478.
79. Lambert E, Carey S. Practice guideline recommendations on perioperative
fasting: a systematic review [published online January 9, 2015]. JPEN J
Parenter Enteral Nutr.

74 Journal of Parenteral and Enteral Nutrition 41(1)
80. Moncure M, Samaha E, Moncure K, et al. Jejunostomy tube feedings
should not be stopped in the perioperative patient. JPEN J Parenter
Enteral Nutr. 1999;23(6):356-359.
81. Pousman RM, Pepper V, Pandharipande P, et al. Feasibility of implement-
ing a reduced fasting protocol for critically ill trauma patients undergoing
operative and nonoperative procedures. JPEN J Parenter Enteral Nutr.
2009;33(2):176-180.
82. McClave SA, Chang, W. When to feed the patient with gastrointestinal
bleeding. Nutr Clin Pract. 2005;20(5):544-550.
83. Heyland DK, Cahill NE, Dhaliwal R, et al. Enhanced protein-energy pro-
vision via the enteral route in critically ill patients: a single center feasibil-
ity trial of the PEPuP protocol. Crit Care. 2010;14(2):R78.
84. Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocalo-
ric feeding and energy balance on clinical outcome in ICU patients. Clin
Nutr. 2005;24(4):502-509.
85. O’Leary-Kelley, Puntillo KA, Barr J, et al. Nutritional adequacy in
patients receiving mechanical ventilation who are fed enterally. Am J Crit
Care. 2005;14(3):222-231.
86. Tepaske R, Velthuis H, Oudemans-van Straaten HM, et al. Clinically rel-
evant differences in accuracy of enteral nutrition feeding pump systems.
JPEN J Parenter Enteral Nutr. 2006;30(4):339-343.
Section 7. Administration: EAD Patency
Background
Feeding tubes (EADs) are prone to clogging for a variety of
reasons. The risk of clogging may result from feeding tube
properties (narrow tube diameter and the tube material), the
tube tip location (gastric vs small bowel), insufficient water
flushes, formula contact with acidic fluid, aspiration for
GRV, contaminated formula, and incorrect medication prep-
aration and administration.
1–3
The importance of maintaining
tube patency is well known. A clogged feeding tube can result
in decreased nutrient delivery or delay administration of medi-
cation, and, if not corrected, the patient may require additional
intervention to replace the tube. Replacement of the EAD can
increase cost and cause patient discomfort. Replacement costs
are higher for jejunal feeding tubes, as they must be replaced in
radiology and require fluoroscopic confirmation of tube
placement.
3
A common cause for an enteral feeding to fail is tube
occlusion, with an incidence as frequent as 23%–35%.
2
In
most cases, tube occlusion delays administration of nutri-
tion support and medications. Prompt restoration of tube
patency reduces the clinical impact and may save health-
care resources devoted to tube replacement. Research sup-
ports water as the best choice for initial declogging efforts.
Flushing a tube with water is an easy and often effective
step. The use of cranberry juice and carbonated beverages
may worsen occlusions because of the acidic pH of these
fluids. Acid can cause proteins in enteral formula to pre-
cipitate within the tube, making the clog worse or leading
to more clogging later on.
2–6
Papain, as a sole treatment
option, has produced varying results.
2
If water does not
work, a pancreatic enzyme solution, an enzymatic declog-
ging kit, or mechanical devices for clearing feeding tubes
are second-line options.
Question 7.1. What are the best practices to maintain
tube patency and prevent tube clogging?
Practice Recommendations
1. Use the largest diameter feeding tube feasible without
sacrificing patient comfort. This includes the largest
inner diameter for a jejunal extension tube through
PEG tubes.
2. Flush feeding tubes immediately before and after
feeding with intermittent feedings. With continuous
feedings, flush at standardized intervals.
3. Flush feeding tubes before and after medication
administration and follow appropriate medication
administration practices (see Section 8).
4. Limit gastric residual checks as acidic gastric contents
may cause protein in enteral formulas to precipitate
within the lumen of the tube.
5. Use aseptic technique when handling enteral formula,
administration sets, and feeding tubes.
6. Use an administration pump when slow rates of enteral
formula are required, such as in the neonatal population,
and respond promptly to pump alarms.
7. Use purified water for flushing the EAD in adult and
neonatal/pediatric population before and after
medication administration.
8. Use purified water for tube flushes in immuno-
compromised or critically ill patients, especially when
the safety of tap water cannot be reasonably assumed.
9. Consider use of an automatic flush pump to prevent
tube clogging and provide additional hydration.
Rationale
Small internal diameter and longer feeding tubes, such as naso-
gastric and nasojejunal tubes, have a higher risk of clogging
compared to shorter and large diameter tubes such as gastros-
tomy tubes.
1–3,7
The larger the diameter of the tube lumen, the
better the flow. Larger bore tubes are less likely to be occluded
by either medication or highly viscous formulas.
3,4,8
In a retrospective review of 560 long-term home EN
patients, Ao et al
9
compared complication rates requiring tube
replacement between jejunostomy tube and PEG tube patients.
The study found that, compared with PEG tubes, jejunostomy
tubes are associated with higher rates of complications that
require tube replacement, with dislodgement and obstruction
being the main causes for tube replacement. Polyurethane
tubes are preferable to silicone because polyurethane better
sustains patency.
10
However, in a laboratory study by Rucart
et al,
11
the impact of different unclogging agents (sterile water,
sodium bicarbonate, papain, digestive enzymes, cola, orange
juice, and pineapple juice) on silicone and polyurethane tubes
showed that silicone tubes seem to be less vulnerable to dam-
age. In this study, only bare tubes were put in contact with the

Boullata et al 75
unclogging agents, and the investigators concluded that occlu-
sion is partly influenced by interactions at the tube surface, and
damage would be aggravated during the administration of EN
and medications.
11
One study found that surface modifications
of polyurethane could reduce both the amount of material
absorbed into the surfaces as well as occlusion in the tubes.
12
The mechanism of clogging may involve denaturation and
precipitation of proteins in enteral formulas when formulas come
into contact with gastric acid from the stomach. Clogging may
also be caused by interactions of the coagulated formula with
feeding tube surfaces, especially during slow feeding infusion
rates.
3,5
It has been observed that feeding tubes positioned in the
highly acidic environment of the stomach may clog more readily
than those positioned in the more neutral pH environment of the
small bowel.
1
The technique of aspirating gastric juices into feed-
ing tubes for GRV checks can increase clogging.
3
The hubs of feeding tubes have been shown to harbor enteric
bacteria that appear to have migrated from GRV and tube patency
checks and further contaminated the extraluminal portion of
enteral delivery sets.
13
Formula contamination must be mini-
mized to prevent clog formation from formula coagulation. To
reduce risk of formula contamination, administer water as sepa-
rate flushes instead of adding it directly to the tube-feeding for-
mula; also, wipe down enteral formula containers with isopropyl
alcohol and allow them to air dry prior to use. Manufacturer rec-
ommended hang times must be followed to prevent bacterial
growth.
1
When handling enteral feeding administration sets, indi-
viduals must follow standard precautions.
Prevention is the preferred way to minimize the risk of
enteral feeding tube clogs. Consistent and scheduled flushing
of all types of tubes during feeding and medication administra-
tion is the best way to decrease the incidence of tube occlusion.
If formula infusion rates are slow, an enteral feeding pump
can be used. Pump occlusion alarms must receive prompt
attention.
3
Enteral feeding pumps with automatic flush systems
are designed to decrease clogged feeding tubes and provide
additional hydration.
14
Flushing the tube is an effective preventive measure. No solu-
tion has been found to be superior to water for its effectiveness,
accessibility, and cost.
3
Based on the available data, water is the
preferred fluid for flushing feeding tubes, reconstituting or dilut-
ing enteral feeding formulas, and diluting medications for enteral
administration. Water used for tube flushing could be drinking
water or sterile water.
15
There are variations in practice, such as
using purified water when the tap water is not proven safe from
microbial or chemical contaminants. In a survey of 823 nurses,
26% always use sterile water to flush tubes before or after medi-
cation administration and 70% always use tap water.
16
Use of
sterile water for tube feeding can minimize the risk of transmis-
sion of pathogens from water sources in high-risk patient areas.
17
In the home setting, either tap water or bottled water is generally
used for water flushes, if tap water is free of contaminants.
In neonatal nutrition, flushes are used sparingly because the
nutrient needs of neonates are so high and there is little room
for fluids that do not contain nutrients. When necessary, flushes
are used at a minimal volume (2 or 3 mL) to maintain the
patency of the feeding tube. In pediatrics, depending on the
size of the child, flushes are more commonly used to maintain
tube patency and to give more water volume.
Question 7.2. What factors determine optimal frequency
and amount of water flushes?
Practice Recommendations
1. In the adult patients, flush feeding tubes with a
minimum volume of 30 mL of water every 4 hours
during continuous feedings or before and after
intermittent feedings.
2. Flush the feeding tube with 30 mL of water after GRV
measurements in an adult patient.
3. In neonatal and pediatric patients, flush feeding tubes
with the lowest volume necessary to clear the tube.
Rationale
There are variations in clinical practice with regards to volume,
timing, and frequency of water flushes. However, consistent
flushing before and after medication administration, bolus feed-
ings, and periodically with continuous or cyclic feedings is very
important to prevent tube occlusion.
18–21
For inpatients receiving
continuous feedings, the amount of water recommended for
flushing ranged from 20–100 mL, and the suggested frequency
of flushing ranged from every 4 hours to every 8 hours. In
patients receiving intermittent or bolus feedings, the amount of
water recommended ranged from 15–100 mL, and sources rec-
ommended flushing both before and after feeding.
18,21
The larger
the flush volume, the more likely the tube is to remain patent;
however, the amount of water used in a flush must be deter-
mined by the patient’s fluid needs and restrictions.
21
In pediatrics, it is important to take the child’s age into
account when flushing an EAD with water. Routine water
flushes are not recommended after each bolus feeding or inter-
rupting continuous feeding for any tubes other than nasojejunal
tubes. For most NG and OG tubes, 3–5 mL of water will suffice
to flush a feeding tube.
1,22
In a recent survey conducted by
ASPEN, 62 clinicians who care for pediatric or neonatal patients
reported using water or air for flushing. The general flushing
volume consensus was to use 2–5 mL in pediatric patients and
1 mL or less of water or air in place of water in neonates.
Question 7.3. What is the best way to open a clogged
feeding tube?
Practice Recommendations
1. Provide proper training, credentialing, and privileging
at the healthcare organizational level to staff responsible
for unclogging tubes according to local practice acts
and institutional privileging.

76 Journal of Parenteral and Enteral Nutrition 41(1)
2. Instill warm water into the EAD using a 30- or 60-mL
syringe, and apply a gentle back-and-forth motion with
the plunger of the syringe.
3. If water flush does not resolve the clog, use an uncoated
pancreatic enzyme solution by crushing one uncoated
pancreatic enzyme tablet and one 325-mg sodium
bicarbonate tablet mixed in 5 mL of water. The solution
should be introduced to the clog and clamp the feeding
tube for at least 30 minutes. If the clog is not cleared
within 30 minutes, the solution should be removed
from the tube and replaced with a fresh mixture.
4. If water flush does not resolve the clog, use an enzyme
containing declogging kit or mechanical declogging
device.
Rationale
Prevention is the best strategy to manage risk of EAD occlu-
sion.
2
However, when EAD occlusion does occur, efforts to
clear the lumen may be appropriate before resorting to EAD
replacement. An approved institutional policy on declogging
enteral feeding tubes will expedite the process. Several
declogging methods are available. The success of the method
has much to do with the cause of obstruction and the knowl-
edge and skill of the provider. The declogging process may
begin when resistance is met when attempting a flush or when
an occlusion alarm sounds on an enteral feeding pump and the
tube is not kinked. However, it may be prudent to begin the
declogging process as soon as the tube becomes sluggish.
3
Warm water is often effective and should be first-line treat-
ment. A 30- or 60-mL syringe is attached initially to the tube
and the plunger pulled back to help dislodge the clog. The flush
syringe is then filled with warm water and reattached to the
tube to attempt a flush. If resistance is met, the plunger of the
syringe may be moved using a gentle back-and-forth motion to
help loosen the clog, then clamp the tube and soak for up to 20
minutes to allow the warm water to penetrate the clog.
3,18
If
resistance continues, a second-line approach is to use an acti-
vated pancreatic enzyme solution.
Pancreatic enzymes have been documented as effective agents
in clearing feeding obstructions caused by enteral formulas.
2,6,23
While enteric-coated and extended-release pancreatic enzyme
products are available, they are not as effective for dissolving
obstructions in feeding tubes.
23,24
Klang
6
evaluated the removal
of the enteric coating to release the pancreatic enzymes for the
purpose of unclogging feeding tubes. The findings show that pan-
creatic enzymes in enteric-coated products can be released and
that these pancreatic enzymes can disrupt clogs. If the clog is of
considerable size, warm water is still the first choice to allow for
the passage of fluid.
23
Another study evaluated the effectiveness
of alkalinized Creon delayed-release pancreatic enzyme protocol
to clear occluded feeding tubes. The protocol was administered to
83 patients, and tube patency was restored to approximately half
(48.2%) of occluded tubes.
5
A recent in vitro study evaluated the
efficacy of an uncoated pancreatic enzyme in comparison to
water or cola in dissolving an enteral formula clog that would
occur in feeding tubes.
6
When combined with sodium bicarbon-
ate, the uncoated pancreatic enzyme effectively dissolved an
enteral formula–associated occlusion, whereas water loosened
the clog but did not dissolve the obstruction, and cola did not
have an impact on the obstruction.
6
Well-designed in vivo studies
are needed to better evaluate all options.
Commercially available enzyme declogging kits contains a
syringe preloaded with a powder of food-grade papain, cellu-
lose, and amylase enzymes, which can break down protein,
fiber, and starch clogs. The powder is activated by pulling
water into the syringe. An elongated hollow catheter is
attached to the syringe so that the declogging solution is deliv-
ered closer in proximity to the clog and left clamped for 30–60
minutes. After that period of time, the tube is unclamped and
a water flush is attempted. If the tube is still clogged, the
declogging process may be repeated with the remaining solu-
tion in the syringe. In one study, 15 of 17 tubes were success-
fully declogged on the first attempt, and the remaining 2 were
declogged on the second try, preventing the need for any tube
reinsertions.
25
Other commercially available mechanical
devices are designed to mechanically dismantle clogs and can
be used for formula or medication-related occlusions.
2,18,24
If
the clog is not resolved by these methods, feeding tube
replacement is indicated.
Topics for Future Research
Identifying incidence rates of EAD obstructions by
unit, institution, and health systems to better understand
the problem
Understand the differences in maintaining tube patency
between recommended guidelines and actual clinical
practice
The safety and efficacy of currently available methods
to unclog enteral feeding tubes
The safety and efficacy of commercially available
agents marketed for resolving occlusions related to
enteral formula or medications
Maximum volume for flushing small bowel feeding
tubes
References
1. Bankhead R, Boullata J, Brantley S, et al. A.S.P.E.N. enteral nutrition prac-
tice recommendations. JPEN J Parenter Enteral Nutr. 2009;33:122-167.
2. Dandeles LM, Lodolce AE. Efficacy of agents to prevent and treat enteral
feeding tube clogs. Ann Pharmacother. 2011;45(5):676-680.
3. Lord LM. Maintaining hydration and tube patency in enteral tube feed-
ings. Safe Pract Patient Care. 2011;5(2):1-11.
4. Magnuson BL, Clifford TM, Hoskins LA, Bernard AC. Enteral nutrition
and drug administration, interactions, and complications. Nutr Clin Pract.
2005;20(6):618-624.
5. Stumpf JL, Kurian RM, Vuong J, Dang K, Kraft MD. Efficacy of a Creon
delayed-release pancreatic enzyme protocol for clearing occluded enteral
feeding tubes. Ann Pharmacother. 2014;48(4):483-487.
6. Klang M. Dissolving a nutrition clog with a new pancreatic enzyme for-
mulation. Nutr Clin Pract. 2013;28(3):410-412.

Boullata et al 77
7. Williams NT. Medication administration through enteral feeding tubes.
Am J Health Syst Pharm. 2008;65:2347-2357.
8. Casas-Augustench P, Salas-Salvado J. Viscosity and flow rate of three high
energy, high fiber enteral nutrition formulas. Nutr Hosp. 2009;24(4):492-497.
9. Ao P, Sebastianski M, Selvaraj V, Gramlich L. Comparison of complica-
tion rates, types, and average tube patency between jejunostomy tubes and
percutaneous gastrostomy tubes in a regional home enteral nutrition sup-
port program. Nutr Clin Pract. 2015;30(3):393-397.
10. Phillips NM, Nay R. A systematic review of nursing administration of
medication via enteral tubes in adults. J Clin Nurs. 2008;17:2257-2265.
11. Rucart PA, Boyer-Grand A, Sautou-Miranda V, Bouteloup C, Chopineau
J. Influence of unclogging agents on the surface state of enteral feeding
tubes. JPEN J Parenter Enteral Nutr. 2011;35(2):255-263.
12. Gaither KA, Tarasevich BJ, Goheen SC. Modification of polyurethane to
reduce occlusion of enteral feeding tubes. J Biomed Mater Res Part B
Appl Biomater. 2009;91B:135-142.
13. Matlow A, Jacobson M, Wray R, et al. Enteral tube hub as a reservoir for
transmissible enteric bacteria. Am J Infect Control. 2006;34:131-133.
14. Jones SA, Guenter P. Automatic flush feeding pumps. Nursing. 1997;
27(2): 56-58.
15. Boullata JI. Enteral nutrition practice: the water issue. Support Line.
2010;32(3):10-17.
16. Guenter P, Boullata J. Drug administration by enteral feeding tube.
Nursing. 2013;43(12):26-33.
17. Denham ME, Kasali A, Steinberg JP, Cowan DZ, Zimring C, Jacob JT.
The role of water in the transmission of healthcare-associated infections:
opportunities for intervention through the environment. Health Environ
Res Design J. 2013;7(suppl):99-126.
18. Fisher C, Blalock B. Clogged feeding tubes: a clinician’s thorn. Pract
Gastroenterol. 2014;38(3):16-22.
19. Schrag SP, Sharma R, Jaik NP, et al. Complications related to percuta-
neous endoscopic gastrostomy (PEG) tubes: a comprehensive clinical
review. J Gastrointest Liver Dis. 2007;16(4):407-418.
20. Blumenstein I, Shastri YM, Stein J. Gastroenteric tube feeding: techniques,
problems and solutions. World J Gastroenterol. 2014;20(26):8505-8524.
21. Reising DL, Neal RS. Enteral tube flushing. Am J Nurs. 2005;105(3):58-63.
22. Lyman BL, Shah SR. Nutrition access. In: Corkins MR, ed. The A.S.P.E.N.
Pediatric Nutrition Support Core Curriculum. 2nd ed. Silver Spring, MD:
American Society for Parenteral and Enteral Nutrition; 2015:567-582.
23. Arriola TAD, Hatashima A, Klang M. Evaluation of extended-release pan-
creatic enzyme to dissolve a clog. Nutr Clin Pract. 2010;25(5):563-564.
24. Bommarito AA, Heinzelmann MJ, Boysen DA. A new approach to
the management of obstructed enteral feeding tubes. Nutr Clin Pract.
1989;4(3):111-114.
25. Lazar J. Treatment of feeding tube occlusions [abstract]. A.S.P.E.N.
Clinical Nutrition Week. Nutr Clin Pract. 2011;26(1):E13.
Section 8. Medication Delivery via Enteral
Access Devices
Background
Medication administration through an EAD can be much more
complex than it would seem. The vast majority of medications are
not formulated to be administered through a feeding tube. In fact,
not all drugs are safe or appropriate for this route of administra-
tion. Careful consideration must be given to each individual medi-
cation prescribed and the goals of therapy. Administration of
many oral medications via enteral feeding tube can be an effective
method of medication administration, but a number of medica-
tions carry complex drug-nutrient or drug-drug interactions that
can impact drug efficacy and drug toxicity. When institutions
create policies regarding medication administration via enteral
feeding tube, they can determine what supplementary order
accompanies the EN order to facilitate proper medication admin-
istration. To prevent untoward consequences, including fatalities,
it may be advisable to have a pharmacist review medication orders
and the preparation of medications for administration via EAD.
It is essential that a necessary medication is appropriately
prepared and administered through a feeding tube without
increasing the risk for complications in the patient. Com-
plications include impairing the patency of the feeding tube,
reducing therapeutic effect of the medication, or increasing
drug toxicity. Additionally, some medications can pose a haz-
ard to the healthcare provider. Safety must be the focus of both
the preparation of the medication and administration of the
medication. Preparation refers to the retrieval of a medication
and any alteration to a dosage form to make it suitable for
delivery through a feeding tube. The alteration may be as sim-
ple as diluting a liquid medication or as complex as compound-
ing a new formulation from multiple components, including
the active pharmaceutical ingredient. The administration step
involves the timing of drug delivery to the patient’s GI tract
with respect to flushing protocols, other medications, and the
EN regimen. Practice recommendations have been available to
practitioners for a number of years.
1
The rationale for such rec-
ommendations has been further described.
2,3
Despite these
publications, recent surveys still identify that some preparation
methods and administration practices do not follow best prac-
tices, which may contribute to adverse risk.
4
Question 8.1. What factors should be evaluated to safely
prepare and administer medication through an
enteral access device?
Practice Recommendations
1. Evaluate factors related to the patient and to their
enteral feeding tube.
a. Identify the patient’s current enteral status (oral,
NPO, or NPO except for medications).
b. Identify whether the patient can take medication by
mouth or requires enteral medication administration.
c. Identify what, if any, anatomical or functional
abnormalities in the patient’s GI tract that may
preclude drug absorption.
d. Document or retrieve the documentation in the
EHR of the current feeding tube so that these data
are available for all healthcare providers. Describe
the device by its entry point and distal end (ie,
nasogastric, percutaneous jejunostomy) and its
diameter (eg, 12 French), rather than by the brand
name or color of the feeding tube.
e. Document or retrieve the documentation in the
EHR, verifying the placement of the distal end of
the feeding tube.
f. Document or retrieve the documentation in the
EHR regarding the feeding formulation and

78 Journal of Parenteral and Enteral Nutrition 41(1)
flushing regimen being administered through the
feeding tube.
g. Confirm tube patency.
2. Evaluate factors related to the medication and its
dosage form.
a. Identify the route (oral vs enteral) and the distal
site of drug administration as ordered (drug
administration should match current enteral status).
b. Develop real-time communications to inform the
pharmacy of any changes to the route or distal site
of medications being prepared and dispensed.
3. Confirm the following aspects of enteral medication
orders and resolve any inappropriate orders with the
prescriber and nurse.
a. The drug dosage form is appropriate for enteral
feeding tube administration (ie, immediate release).
i. Avoid any solid dosage form medications
that would result in a significant change in
the absorption of the active ingredient(s) if
opened (capsule) or crushed (tablet).
ii. Evaluate each medication for its inherent
solubility and release characteristics. If
crushing the medication alters its delivery
(eg, enteric coated, extended release, or novel
excipients for alternative delivery systems),
consider an alternative dosage form, drug, or
route of administration.
b. The drug and the formulation are both appropriate
based on the distal end of the feeding tube.
i. Avoid bypassing the primary site of drug
absorption.
ii. Avoid high-osmolality or highly viscous
preparations.
c. Any medication order that will require a
preparation step (eg, crushing, diluting, mixing)
prior to administration is identified.
i. Establish and follow organizational policies
and procedures to prepare medications for
enteral administration that will comply with
USP chapter <795>.
4. Document in the EHR any clarifications or interventions
related to the prescribing, reviewing, preparation, or
administration of medications for the patient receiving
EN.
5. Work with CPOE vendors and application architects to
design systems such that each medication is ordered by
the appropriately intended route of administration.
Rationale
The enteral route of drug administration is unique and differs
from the oral administration in several ways. The topic of enteral
medication administration is receiving more attention in the lit-
erature.
5
Organizations that develop and put into practice proto-
cols for enteral medication administration are much more likely
to prevent related medication errors than those that lack proto-
cols.
6,7
Best practices include an evaluation of both the EAD and
the medication profile in a systematic fashion. Although not
widely recognized or reported, medication errors related to the
enteral route of administration happen.
8
Inappropriate prepara-
tion and/or administration technique can lead to an occluded
tube, reduced drug effect, or increased drug toxicity. These poten-
tial adverse outcomes are not always captured in medication error
rates. Routine reporting of all enteral medication errors to the
medication safety committee or other appropriate institutional
committee is important so that systems improvements can be
made to address them. The responsibility for preventing enteral
medication errors should be shared by the prescriber, pharmacist,
and nurse. The nurse is in a difficult position if a prescriber enters
an inappropriate medication order and the pharmacist does not
clarify it. An interdisciplinary group of healthcare providers,
including knowledgeable prescribers, pharmacists, and nurses,
can work together to develop protocols for administering medi-
cations through enteral tubes within their organization.
8
Summary
documentation of clarifications or interventions related to medi-
cation use in the enterally fed patient can be reviewed on a regu-
lar basis. Unless a culture of safety already exists within an
organization to consider, document, and report all errors related
to EN and medication administration in patients receiving EN,
institutions may assume that there are no safety issues or errors.
Emami et al
9
reported a case of a 53-year-old man who was
in the ICU and subsequently intubated with a nasogastric tube
being used for nutrition support and medication administra-
tion. During his 30-day hospitalization, this patient improperly
received all of his scheduled oral medications via feeding tube,
including multiple sustained-release and extended-release
drugs, crushed and combined with each other in 40 mL of tap
water. A multidisciplinary team with a pharmacist reviewing
medications to be administered via enteral feeding tube could
have prevented these errors and intervened with proper recom-
mendations for medication administration.
Caregivers are typically confident that they prepare and/or
administer drugs appropriately, although surveys have suggested
otherwise.
1,4
Prospective observational studies suggest that these
types of medication errors may occur with about 60% of doses;
these finding highlight the need for pharmacists to be vigilant and
work closely with prescriber and nurse colleagues.
10,11
In a study
by Boullata and colleagues,
12
patients with “NPO” orders and
unable to take medication by mouth were still prescribed drugs
“PO” over 80% of the time. Of those, many orders were not cor-
rected by the pharmacist reviewing the orders, which therefore
placed the nurse in the precarious position of committing a wrong
route medication error. In this same study of drug administration
in enterally fed hospitalized patients, less than 20% of drugs
administered directly into the small bowel were considered
appropriate.
12
In these cases, therapeutic alternatives or a differ-
ent route of administration may need to be considered.
A number of medications, including modified-release dos-
age forms (eg, delayed release, sustained release), are inappro-
priate for the enteral route. A listing of the many oral dosage

Boullata et al 79
forms that should not be crushed or opened is readily avail-
able.
13
These dosage forms are implicated not only in potential
for interaction or excessive bolus drug doses but also for
exposing caregivers (including via inhalation) to allergenic,
cytotoxic, and teratogenic products.
Liquid medications, while offering the advantage of an easier
to administer dosage form for the feeding tube, rarely are the ideal
formulation for that route. Ingredients such as sugars, preserva-
tives, and thickening agents can cause significant side effects that
may be interpreted as intolerance to the EN formula. The undi-
luted administration of liquid medications with an osmolality
>500–600 mOsm/kg may be associated with significant GI distur-
bances, especially in vulnerable patients.
14–16
The healthy stomach
is able to tolerate hyperosmolar liquids much better than the small
intestine, although gastric emptying may be delayed. Gastric
administration reflects the intended site of drug disintegration
when administered orally, but postpyloric administration may
alter bioavailability and affect the risk of GI complaints and mal-
absorption. The highest physiologic osmolality expected in the
small bowel is ~600 mOsm/kg in the fed jejunum.
Crushing an immediate-release solid dosage form and giving
the medication with adequate dilution is often the safest course of
action. For a medication administered enterally to be absorbed, it
must first be dissolved in solution. Preparing a liquid dosage form
is fraught with complexities, and an appropriate reference should
be consulted before assuming stability.
17–21
Even simple opera-
tions such as crushing an immediate-release tablet and mixing it
with water must be examined because the safety and efficacy of
the medication may be affected by the quality of the water (tap vs
purified), degree of crushing (particle size), and time of exposure
(as the drug may degrade in a liquid form). In the absence of drug-
specific data, pharmaceutical principles should be used to make
recommendations. Many newer drugs have poor water solubility.
They are formulated with coprecipitates, solubilizers, and surfac-
tants that should remain in close proximity to the active pharma-
ceutical ingredient (API) to ensure dissolution at the targeted time.
Mixing these formulations in a separate vessel and then transfer-
ring the contents to the enteral feeding system may allow the sepa-
ration of ingredients, precipitation of the API, and failure to
absorb. Suggestions for drug products containing a poorly soluble
API have included placing the product in the barrel of a syringe
and adding a diluent to allow a slurry to form before administra-
tion. This methodology requires a larger EAD (14 Fr).
By addressing issues with drug administration in the patient
with an EAD, the pharmacist plays a critical role in supporting the
prescriber and the nurse.
22
When evaluating an enteral drug order
in a patient receiving EN, the pharmacist needs to be aware of the
patient’s GI status, the EN regimen, and the location of the EAD
to identify inappropriate administration routes, any potential inter-
actions, or other administration route issues. The pharmacist must
resolve conflicts when a prescriber orders a drug to be adminis-
tered PO in the patient with NPO orders. If the drug is intended for
administration through the EAD, the order must indicate this
route; if it does not, the nurse who administers the medication by
the “wrong route” seems to commit a medication error. More
important, the pharmacist needs to decide whether the drug and its
formulation are appropriate for EAD administration. Given the
risks for physicochemical incompatibility and instability, drugs
are not to be admixed together. Potential drug-nutrient interactions
that result from a physical, chemical, physiologic, or pathophysi-
ologic relationship between a drug and EN also need to be consid-
ered.
1
An interaction is considered clinically significant when it
influences therapeutic response (or compromises nutrition status)
with clinical consequences related to altered drug (or nutrient) dis-
position. For example, EN can alter drug bioavailability. The bio-
availability of some drugs may benefit from administration in
close proximity to EN, whereas the bioavailability of others may
be significantly reduced in the same circumstances. In the latter
case, administration of drug should be temporally separated from
EN. Lists of drugs to be administered with, or separated from, EN
are best used in conjunction with other considerations for drug
administration via EAD (eg, flushing protocol, appropriate drug
dilution, location of EAD distal tip).
Question 8.2. What steps offer the safest method to
deliver medication through an enteral feeding tube?
Practice Recommendations
1. Develop policies and procedures to ensure safe
practices by staff across all departments involved with
enteral medication preparation and administration.
2. Identify drug, dose, dosage form, route (ie, enteral),
and access device (eg, nasoduodenal tube) in the
prescriber’s order.
3. Review by a pharmacist of each medication order to
determine whether the enterally administered medi-
cation will be safe, stable, and compatible as ordered.
4. Institute and follow nursing policies and procedures to
prepare and administer each medication safely.
5. Provide nonsterile compounding pharmacy services
to support medication preparation.
6. Use best practices as per USP <795> for any enteral
drug preparations compounded in advance (ie, not for
immediate use) and these should be based on:
a. Published stability data and clearly described
with citations in the organization’s Master
Formulation Records
b. Documenting in a permanent Compounding Record
c. Providing a beyond-use date
d. Storage in a container consistent with the
stability/compatibility literature and USP <795>
7. Do not add medication directly to an enteral feeding
formula.
8. Administer each medication separately through an
appropriate access.
9. Avoid mixing together different medications intended
for administration through the feeding tube given the
risks for physical and chemical incompatibilities, tube
obstruction, and altered therapeutic drug responses.

80 Journal of Parenteral and Enteral Nutrition 41(1)
10. Use available liquid dosage forms only if they are
appropriate for enteral administration. If liquid dosage
forms are inappropriate or unavailable, substitute only
immediate-release solid dosage forms.
11. Prepare approved immediate-release solid dosage forms
of medication for enteral administration according to
pharmacist instructions. Techniques may include:
a. Crush simple compressed tablets to a fine powder
and mix with purified water.
b. Open hard gelatin capsules and mix powder
containing the immediate-release medication
with purified water.
12. Use only appropriate instruments to measure and
prepare enteral medication.
13. Use only clean enteral syringes (20 mL with ENFit
device) to administer medication through an EAD.
14. Provide appropriate tube irrigation around the timing
of drug administration:
a. Prior to administering medication, stop the feeding
and flush the tube with at least 15 mL water.
b. Administer the medication using a clean enteral
syringe.
c. Flush the tube again with at least 15 mL water,
taking into account the patient’s volume status.
d. Repeat with the next medication.
e. Flush the tube one final time with at least 15 mL
water.
15. Restart the feeding in a timely manner to avoid
compromising nutrition status. Hold the feeding by 30
minutes or more only if separation is indicated to
avoid altered drug bioavailability.
16. Consult with an adult or pediatric pharmacist for patients
who receive medications coadministered with EN.
Rationale
The most consistent delivery of medication through an EAD
comes from adequate drug dilution and flushing.
23–26
Medication that is in an appropriately powdered form, either
from pulverized tablets, capsule contents, or dry powder prod-
ucts intended for reconstitution, needs to be diluted to ensure
delivery through the EAD. Dilution may be necessary for the
enteral administration of liquid medications (ie, solutions, sus-
pensions) to reduce viscosity or osmolality. Reducing viscosity
allows the full drug dose to reach the distal end of the EAD,
especially for longer, small-bore tubes. Not diluting a suspen-
sion could result in a significant decrease in drug delivery and
bioavailability when administered through an EAD.
23,24
In a
crossover study in healthy volunteers, administration of an
undiluted drug suspension (posaconazole) through a 16 Fr
nasogastric tube resulted in a 23% lower bioavailability than
the oral administration.
23
In another study, compared to 1:1
(v:v) dilution, 11%–24% of undiluted drug suspension (carba-
mazepine) was lost, depending on the diluent, despite all 12 Fr
nasogastric tubes being flushed.
24
Again, the most consistent delivery through an EAD comes
from adequate drug dilution and flushing.
23,24
Water is the sim-
plest fluid for diluting powdered or liquid medication (see
Appendix 1). The U.S. Pharmacopeia requires that purified water
be used for preparation of drug dosage forms. Purified water
refers to water that is free of contaminants (chemical and biologi-
cal) following source water selection, distillation, and filtration.
25
Drinking water (tap, bottled, and well water) may contain
chemical contaminants. Therefore, the use of drinking water to
dilute medication prepared for enteral administration can increase
the risk for potential drug interactions, which may, in turn, alter
drug bioavailability.
26,27
For this reason, purified water is required.
Sterile water for irrigation is an example of a purified water prod-
uct, but there is no need for the diluent to be sterile.
26
However, the
potential for acute drug-drug, drug-chemical interactions when
contaminated waters are combined with medication has not been
quantified. More data regarding the appropriateness of medication
dilution and the potential for drug interactions are needed.
Dilution with 30–60 mL of water seems adequate for pow-
dered medication.
28–31
The volume required to dilute liquid med-
ication depends on the desired degree of viscosity and/or
osmolality. Diluting viscous suspensions in a volume of at least
1:1 seems to be adequate for some drugs.
24,32
High-osmolality
medication can result in localized adverse effects at the mucosa
or create an osmotic effect throughout portions of the bowel. The
higher the osmolality, the greater the volume of diluent required
to lower the osmolality.
31
The case has been made that it would
be more practical to crush an acetaminophen tablet to a fine
powder and disperse in a smaller volume of water than to use a
liquid formulation that requires significant volume dilution.
21
The time lapse between stopping the EN, administering the
drug, and restarting the EN will depend on any potential for
drug-nutrient interaction in the GI lumen.
1,33
When an immediate-release solid dosage form needs to be
prepared for EAD administration, there are several options
available. A study compared 3 preparation methods in terms of
crushing yield, microscopic observation, suspension stability,
and aerosolization.
34
The data suggest no significant difference
in efficiency of crushing between methods. However, the parti-
cle granularity differed by drug, indicating that crystal structure
and excipients are a factor in determining how fine a powder can
be produced, and this may predict interaction potential. Confined
crushing yielded smaller particles, but all particles suspended
similarly in water. Open crushing yielded significantly greater
amounts of particulate matter (>10
6
particles) in the cubic meter
of space directly above where the drug is prepared.
34
Although more time-consuming, separation of each medica-
tion administered through an EAD reduces the risk of tube
obstruction and interactions. Drug errors can additionally be
related to inappropriate use of dosing instruments as well as
health literacy.
35
The ideal medication delivery system for EN administration
would be to have the drug fully dissolved in a solution that has
a neutral pH, tolerable osmolarity, and low viscosity. This
delivery system does not exist. Most liquids are thick and

Boullata et al 81
contain sweeteners and insoluble excipients. The drugs that are
not available in liquid formulation have limited stability in that
state, making them poor candidates for enteral administration.
To optimize patient safety, it is important to evaluate each
medication for appropriateness of the specific enteral route.
Factors to assess include the formulation, solubility of the API,
and the use of excipients to deliver the medication. Each medi-
cation is unique, and global assumptions can lead to both inef-
fectiveness of the targeted medications or toxicity of enhanced
absorption.
One approach that is becoming popular
21
:
1. Open an appropriate oral or feeding tube syringe by
withdrawing the plunger.
2. Place the single medication dosage form in the syringe.
This could be a capsule or tablet.
3. Replace plunger.
4. Add 15–30 mL purified water at room temperature.
5. Wait 20 minutes for slurry to form.
6. Once all solids have disintegrated, use the syringe to
administer the medication through a flushed feeding tube.
7. Rinse the feeding tube with an additional 15–30 mL
purified water.
This method is especially useful when handling hazardous
medications ordered for administration through an EAD. It is also
beneficial in administering drugs formulated with cosolvents that
would precipitate if mixed in a separate container and transferred
to a syringe. Some capsules are slow to dissolve in water and will
form clogs in smaller bore feeding tubes (<12 Fr). In these cases,
a special compounded liquid formulation will be needed.
Extra caution is needed when using specialty pharmacies
that compound oral liquid formulations. Most formulations
contain high amounts of sorbitol, a potent cathartic, which is
added as a sweetening agent. There is no need for sweeteners
and flavoring agents for drugs to be administered through an
EAD. Ask the compounding pharmacist to use a low-osmolar-
ity suspending agent for compounding drugs that will be more
likely tolerated for feeding tube use.
Question 8.3. When prescribing medications to be
administered via an enteral route, what is the safest
and most effective way to name (communicate) the
route and site of administration?
Practice Recommendations
1. Develop policies, procedures, and practices across
departments for a uniform way of referring to short-
term and long-term EADs across departments.
2. Describe each device by its entry point (eg, naso-,
percutaneous) and its distal end (ie, gastric, jejunostomy),
its diameter (eg, 12 French). Avoid references to brand
names, shapes, or colors of the feeding tube.
3. Work with EHR vendors so that CPOE systems allow
for a specific route of administration when medications
are intended for feeding tube administration.
Rationale
The method used to prepare a drug for administration through
an EAD depends on the internal diameter, total internal sur-
face area, and distal site of drug delivery. The dimensions of
the feeding tube are crucial in determining the appropriate
selection of a formulation for the medication to be adminis-
tered. Tubes with an internal diameter equal to or greater than
10 Fr work best for administering crushed or dissolved solid
dosage medications. If the tube is less than 10 Fr, the medica-
tion must be in a liquid formulation with few, if any, residual
solids. Some feeding tubes have internal dual channels to
allow for the separation of stylus. Due to their small internal
diameters, these tubes are especially prone to clogging.
The proximal site or entry point provides a clue as to the
length of the tube and its overall surface area. Longer tubes are
more prone to complications related to the cumulative resistance
to flow as well as medication interaction with the tubing mate-
rial. The distal site, whether gastric, duodenal, or jejunal, will
determine the extent of dissolution that will be required in the
formulation selection. For a jejunal feeding tube, the API should
be fully dissolved and prepared in a vehicle with a final osmolar-
ity of approximately 285 mOsm/L. In some cases, acid is added
to the formulation to enhance API solubilization; in other cases,
sodium bicarbonate is added to remove the medication’s enteric
coating and allow dissolution. Liquid medications administered
into a feeding tube with the distal end placed in the stomach can
have a higher osmolarity of approximately 500 mOsm/L. Drugs
administered into the stomach can include slurries and suspen-
sions since dissolution should occur in the gastric fluid.
It is troubling how few CPOE systems allow for selecting
the specific feeding tube route as a designation when placing an
electronic medication order for that route. The practice of using
“PO” for the “allowed” route and then indicating special admin-
istration instructions in a free text field can contribute to error.
Question 8.4. What factors will determine whether the
pharmacy or the nurse will prepare medication for
enteral administration?
Practice Recommendations
1. Develop policies, procedures, and practices to
determine how the workload of drug preparation for
enteral administration will be distributed.
2. Only trained personnel can prepare hazardous
medications or drugs that contain known allergens.
Prepare these medications under conditions that protect
all personnel, in compliance with OSHA and NIOSH
publications as well as USP Chapter <800>.
3. Avoid environmental risks of cross-contamination
between medications.
4. If permitted by the organization, a nurse may prepare
nonhazardous and nonallergenic drugs with limited
stability in a clean area of the medication room.
a. Maintain practices and responsibilities consistent
with USP chapter <795>.

82 Journal of Parenteral and Enteral Nutrition 41(1)
b. Unless prepared for immediate use, label the final
container (eg, enteral syringe) according to all
applicable federal and state laws, and include a
beyond-use date.
5. Appropriately trained personnel in the pharmacy are
responsible for preparing drugs that require significant
manipulation and fall within the context of compounding.
a. Follow the organization’s policies and procedures
as well as USP chapter <795>.
b. Unless prepared for immediate use, label the final
container (eg, enteral syringe) according to all
applicable federal and state laws, and include a
beyond-use date.
Rationale
Ideally, all medications that must be compounded or mixed
are presented to the nurse in the final dosage formulation to be
administered. However, each organization will need to deter-
mine how best to approach this given its resources and admin-
istrative support structures.
The enteral administration of hazardous medications
poses additional risk. For oral chemotherapy agents, crushing
must be avoided. Ideally, a closed-system transfer device,
similar to those used for reconstitution of injectable chemo-
therapy, would be used in compounding of chemotherapy for
enteral administration. For more information on hazardous
medication handling, refer to OSHA and USP chapter <800>
guidance.
36,37
Several reports indicate that the optimal way to prepare
drugs is to place the drug inside a syringe, add water, and then
mix to dissolve the medication. This method can be safer than
crushing drugs because it limits the operator’s exposure to
some of the hazardous components of the tablet or capsule for-
mulation. Furthermore, putting the uncrushed drug in a syringe
and making a slurry keeps all components together and allows
delivery similar to oral intake of the medication. Many drugs
have poor water solubility and are formulated with excipients
to enhance dissolution. Crushing the drug and mixing in a sep-
arate container risks that the components will separate and
drug will precipitate.
Question 8.5. What is the optimal method for the
pharmacy to dispense medications to the patient
care unit for the nurse to administer via the feeding
tube?
Practice Recommendations
1. Develop policies and procedures that describe the
labeling and dispensing of enteral medications in a
manner consistent with federal and state laws as well
as USP chapter <795>.
2. Label the medication using:
a. Patient identifiers
b. Common (ie, generic) name of the drug using
TALLman lettering as indicated
c. Dose of the drug
d. Scheduled date/time of the dose
e. Manufacturer name and lot number
f. Beyond-use date
g. The organization’s Compounding Record number,
if compounding was required
3. Affix the label to the container being dispensed (eg, the
enteral syringe).
4. Dispense the medication in a manner consistent with
organizational staffing.
a. Dispense the commercial unit dose product for the
nurse to prepare.
b. Dispense unit doses prepared in the pharmacy in a
suitable container.
i. Dispense dry powder medication with
directions to dilute it with purified water.
ii. Dispense a stable slurry of the dry powder
medication in an appropriate diluent.
iii. Dispense a commercially available liquid or
extemporaneously compounded liquid
medication with directions to dilute it further
or administer it as is.
5. Dispense medication with appropriate directions for
preparation and administration.
Rationale
To optimize safety, all medication dispensed for enteral
administration must reflect practices related to stability and
be appropriately labeled. Each medication must be com-
pounded into a unique formulation appropriate for the spe-
cific type of EAD administration. Careful adherence to the
recommendations of USP <795> is essential to ensure the
medication reaches its intended target.
38
Even simple opera-
tions, such as crushing an immediate-release tablet and mix-
ing it with water, must be examined because outcomes may
be influenced by the quality of the water (tap or purified),
degree of crushing (larger particles are more likely to clog
EADs), or the time of exposure (the drug may degrade in a
liquid form).
Many newer drugs have poor water solubility. They are
formulated with coprecipitates, solubilizers, and surfactants
that should remain in close proximity to the API to ensure
dissolution at the targeted time. In some cases, mixing these
formulations in a separate vessel and then transferring the
contents to the syringe allows the ingredients to separate or
the API to precipitate, which decreases absorption of the API.
It is advisable to prepare all poorly soluble API drugs in a
syringe, adding the diluent and allowing a slurry to form
before administration. This methodology requires large-bore
feeding tubes (14 French) for adults.

Boullata et al 83
Question 8.6. What medications are of particular
concern for enteral delivery?
Practice Recommendations
1. Maintain at the healthcare organizational level a list of
medications that pose a concern for administration via
EAD.
2. Ensure that this list of medications complies with
NIOSH guidelines, OSHA regulations, and USP
chapter <800> and reflects the release characteristics
of the drug dosage form as well as data on interactions
with EN formula and/or enteral feeding tubes.
Rationale
Safe handling of hazardous medications is imperative.
According to USP chapter <800>, NIOSH guidelines, and
OSHA regulations, when a hazardous medication is assigned
for feeding tube administration and a liquid formulation is not
available, the dosage form must not be crushed outside the
confines of a biological safety cabinet.
36,37,39
An alternative to
crushing is to place the intact hazardous medication in a syringe
and add water to dissolve.
40
Another important safety issue is
the release character of the drug dosage form. For example, the
crushing of extended-release medications can cause the entire
dose to be released immediately. Significant adverse effects,
including fatality, can result from this bolus administration.
41
A significant number of drugs are not to be administered
through an EAD. These include hazardous drugs as well as
some nonhazardous drugs. The number of drugs considered
“hazardous” will vary with the practice setting, and the list will
be determined by the experts within each organization. The
characteristics of hazardous drugs include those that are carci-
nogenic, mutagenic, or teratogenic or that impair fertility, as
well as those causing serious toxicity at low doses in treated
patients.
36,37,39
For the purposes of enteral administration, non-
hazardous drugs that would nonetheless be inappropriate to be
manipulated and administered through an EAD are also impor-
tant to incorporate into the list and include the modified-release
versions of medication.
13
Lists of these substances can be read-
ily accessed through NIOSH (http://www.cdc.gov/niosh/
docs/2014-138/pdfs/2014-138_v3.pdf
) and ISMP (http://www.
ismp.org/Tools/DoNotCrush.pdf
) for review by each organiza-
tion in generating their own inventory.
13,39
The list should be
periodically reviewed and updated to reflect new data and mar-
keted drug products added to an organization’s formulary.
The administration of drugs via a feeding tube can clog the
tube, which may lead to serious complications for patient care.
Clogs also form as a reaction of protein with the acidity of the
gastric environment. The appropriate method for fixing the
clog may depend on what caused it. Many drugs are a weak
base and dissolve better in acidic fluid, whereas protein
clumps in acid and responds better to protease enzymes.
Flushing the tube with water has been shown to work well for
both forms of clogs.
Many drugs require the acid environment of the stomach to
properly dissolve. Once they are dissolved, they are absorbed
in the more basic milieu of the small bowel. Administration of
these drugs directly into the jejunum will result in altered
absorption and changes in efficacy.
42
Each medication admin-
istered through a feeding tube with the distal site past the pylo-
rus must be evaluated for changes in absorption by that
route.
43,44
Consideration must also be given to the action of
gastric acid–inhibiting treatments, such as proton pump inhibi-
tors, which may also alter absorption of medications.
The number of errors associated with the use of enteral-
based medications is cause for concern.
45
Once syringes with
enteral connectors, such as the ENFit design, are fully avail-
able, their use to dispense medications prepared for feeding
tube administration may improve safety.
46
The ENFit design
only allows for feeding tube administration and cannot to be
confused with any other type of therapeutic access in the patient.
The administration of medications with poor solubility is
another challenge in caring for patients on EN. These medica-
tions are specially formulated to allow dissolution under optimal
conditions.
47
If these drugs are mixed in a container and allowed
to sit, the excipients will separate from the active drug and pre-
cipitate out. Administering medication that has separated from
the excipients can possibly lead to therapeutic failure.
48
Therefore, drugs with poor water solubility should not be com-
pounded into a liquid formulation unless they are evaluated for
clinical efficacy as well as stability. In some cases, the drug can
be combined with water in an oral syringe and administered
immediately after forming a slurry.
Topics for Further Research
Identify incidence rates of enteral medication
preparation and administration errors to better
understand the problem
Preparation of specialized formulations for feeding tube
administration
Design a suspension vehicle with a low osmolarity
(~285 mOsm/L) and neutral pH (~6.5–7.5)
Stability of oral medications in low-osmolar suspending
agents and/or water
Prospective description of enteral drug preparation and
administration systems such as type of water, degree of
crushing, time to decomposition, EAD French, or
surface area
References
1. Bankhead R, Boullata J, Brantley S, et al; A.S.P.E.N. Board of Directors.
Enteral nutrition practice recommendations. JPEN J Parenter Enteral
Nutr. 2009;33:122-167.
2. Boullata JI. Drug administration through an enteral feeding tube: the ratio-
nale behind the guidelines. Am J Nurs. 2009;109(10):34-42.

84 Journal of Parenteral and Enteral Nutrition 41(1)
3. Klang M. Recommendations for compounding medications for feeding
tube administration. Int J Pharm Compd. 2010;14(4):276-282.
4. Guenter P, Boullata J. Drug administration by enteral feeding tube.
Nursing. 2013;43(12):26-35.
5. Kurien M, Penny H, Sanders DS. Impact of direct drug delivery via gastric
access devices. Expert Opin Drug Deliv. 2015;12:455-463.
6. van den Bemt PMLA, Fijn R, van der Voort PHJ, Gossen AA, Egberts
TCG, Brouwers JRBJ. Frequency and determinants of drug administration
errors in the intensive care unit. Crit Care Med. 2002;30:846-850.
7. van den Bemt PMLA, Cusell MBI, Overbeeke PW, et al. Quality improve-
ment of oral medication administration in patients with enteral feeding
tubes. Qual Saf Health Care. 2006;15:44-47.
8. Institute of Safe Medication Practices. Preventing errors when administer-
ing drugs via an enteral feeding tube. ISMP Acute Care Medication Safety
Alert Newsletter. May 6, 2010. https://www.ismp.org/newsletters/acutec-
are/articles/20100506.asp. Accessed May 12, 2016.
9. Emami S, Hamishehkar H, Mashayekhi, S, et al. Errors of oral medication
administration in a patient with enteral feeding tube. J Res Pharm Pract.
2012;1(1):37-40.
10. Bertsche T, Niemann D, Mayer Y, et al. Prioritizing the prevention of
medication handling errors. Pharm World Sci. 2008;30:907-915.
11. Joos E, Mehuys E, Van Bocxlaer J, et al. Drug administration via
enteral feeding tubes in residential care facilities for individuals with
intellectual disability: an observational study. J Intellect Disab Res.
2015;59:215-225.
12. Boullata J, Aloupis M, Fodero K, et al. Appropriateness of drug administra-
tion in enterally fed patients [abstract]. Crit Care Med. 2009;37(suppl):A122.
13. Mitchell JF. Oral dosage forms that should not be crushed, 2015. http://
www.ismp.org/Tools/DoNotCrush.pdf
14. Niemiec PW Jr, Vanderveen TW, Morrison JI, Hohenwarter MW.
Gastrointestinal disorders caused by medication and electrolyte solu-
tion osmolality during enteral nutrition. JPEN J Parenter Enteral Nutr.
1983;7(4):387-389.
15. Atakent Y, Ferrara A, Bhogal M, Klupsteen M. The adverse effects of
high oral osmolal mixtures in neonates. Clin Pediatr. 1984;23:487-491.
16. Obladen M, Mutz A. Oral medications in preterm infants? Physical
properties of liquid drugs [in German]. Monatsschr Kinderheilkd.
1985;133:669-674.
17. Glass BD, Haywood A. Stability considerations in liquid dosage forms
extemporaneously prepared from commercially available products. J
Pharm Pharmaceut Sci. 2006;9:398-426.
18. Allen LV. The Art, Science, and Technology of Pharmaceutical Compound-
ing. 4th ed. Washington, DC: American Pharmacists Association; 2012.
19. Trissel LA. Trissel’s Stability of Compounded Formulations. 5th ed.
Washington, DC: American Pharmacists Association; 2012.
20. Jackson M, Lowey A. Handbook of Extemporaneous Preparation: A Guide
to Pharmaceutical Compounding. London, UK: Pharmaceutical Press; 2010.
21. White R, Bradnam V. Handbook of Drug Administration via Enteral
Feeding Tubes. 3rd ed. London, UK: Pharmaceutical Press; 2015.
22. Langebrake C, Hilgarth H. Clinical pharmacists’ interventions in a
German university hospital. Pharm World Sci. 2010;32:194-199.
23. Ashley ESD, Varkey JB, Krishna G, et al. Pharmacokinetics of posacon-
azole administered orally or by nasogastric tube in healthy volunteers.
Antimicrob Agents Chemother. 2009;53:2960-2964.
24. Clark-Schmidt AL, Garnett WR, Lowe DR, Karnes HT. Loss of carbam-
azepine suspension through nasogastric feeding tubes. Am J Hosp Pharm.
1990;47:2034-2037.
25. United States Pharmacopeia. USP <1231> Water for pharmaceutical pur-
poses. http://www.usp.org. Accessed August 4, 2016.
26. Boullata JI. Enteral nutrition practice: the water issue. Support Line.
2010;32(3):10-17.
27. Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD,
Snyder SA. Pharmaceuticals and endocrine disrupting compounds in U.S.
drinking water. Environ Sci Technol. 2009;43:597-603.
28. Healy DP, Brodbeck MC, Clendening CE. Ciprofloxacin absorption is
impaired in patients given enteral feedings orally and via gastrostomy and
jejunostomy tubes. Antimicrob Agents Chemother. 1996;40:6-10.
29. Cohn SM, Sawyer MD, Burns GA, Tolomeo C, Milner KA. Enteric
absorption of ciprofloxacin during tube feeding in the critically ill. J
Antimicrob Chemother. 1996;38:871-876.
30. Yuk JH, Nightingale CH, Sweeney KR, et al. Relative bioavailability in
healthy volunteers of ciprofloxacin administered through a nasogastric
tube with and without enteral feeding. Antimicrob Agents Chemother.
1989;33:1118-1120.
31. Rollins CJ. Drug-nutrient interactions in patients receiving enteral nutri-
tion. In: Boullata JI, Armenti VT, eds. Handbook of Drug-Nutrient
Interactions. 2nd ed. New York, NY: Humana Press; 2010:367-410.
32. Seifert CF, McGoodwin PL, Allen LV. Phenytoin recovery from percu-
taneous endoscopic gastrostomy Pezzer catheters after long-term in vitro
administration. JPEN J Parenter Enteral Nutr. 1993;17:370-374.
33. Wohlt PD, Zheng L, Gunderson S, Balzar SA, Johnson BD, Fish JT.
Recommendations for the use of medications with continuous enteral
nutrition. Am J Health Syst Pharm. 2009;66(16):1458-1467.
34. Salmon D, Pont E, Chevallard H, et al. Pharmaceutical and safety consid-
erations of tablet crushing in patients undergoing enteral intubation. Int J
Pharmaceut. 2013;443:146-153.
35. Yin HS, Mendelsohn AL, Wolf MS, et al. Parents’ medication administra-
tion errors. Arch Pediatr Adolesc Med. 2010;164:181-186.
36. Occupational Safety and Health Administration, U.S. Department of
Labor. OSHA Technical Manual, Section VI, Chapter 2: controlling occu-
pational exposure to hazardous drugs. https://www.osha.gov/dts/osta/otm/
otm_vi/otm_vi_2.html. Accessed May 12, 2016.
37. United States Pharmacopeia. USP <800> Hazardous drugs—handling in
healthcare settings. November 20, 2013. http://www.usp.org/sites/default/
files/usp_pdf/EN/m7808.pdf. Accessed May 12, 2016.
38. United States Pharmacopeia. USP <795> Pharmaceutical compounding—
nonsterile preparations. January 1, 2014. http://www.usp.org/sites/default/
files/usp_pdf/EN/gc795.pdf. Accessed May 12, 2016.
39. National Institute for Occupational Safety and Health, Centers for Disease
Control and Prevention, U.S. Department of Health and Human Services.
NIOSH list of antineoplastic and other hazardous drugs in healthcare
settings, 2014. Publication No. 2014-138. http://www.cdc.gov/niosh/
docs/2014-138/pdfs/2014-138_v3.pdf. Accessed May 12, 2016.
40. Wakui N, Ookubo T, Iwasaki Y, Ito R, Saito K, Nakazawa H. Development
of a closed drug preparation method for oral anticancer drugs. J Oncol
Pharm Pract. 2013;19(4):315-320.
41. Cleary JD, Evans PC, Hikal AH, Chapman SW. Administration of crushed
extended-release pentoxifylline tablets: bioavailability and adverse
effects. Am J Health Syst Pharm. 1999;56(15):1529-1534.
42. McIntyre CM, Monk HM. Medication absorption considerations in
patients with postpyloric enteral feeding tubes. Am J Health Syst Pharm.
2014;71(7):549-556.
43. Nelson EB, Abernethy DR, Greenblatt DJ, Ameer B. Paracetamol
absorption from a feeding jejunostomy. Br J Clin Pharmacol.
1986;22(1):111-113.
44. Nelson EB, Levitt JR. Aspirin absorption from a feeding jejunostomy. J
Am Geriatr Soc. 1980;28(12):556-557.
45. Guenter P, Hicks RW, Simmons D. Enteral feeding misconnections: an
update. Nutr Clin Pract. 2009;24(3):325-334.
46. TJC: plan and prepare for the transition to new tubing connectors to
minimize the risk of dangerous misconnections, clinician frustration. ED
Manag. 2014;26(12):Suppl 1-3.
47. Truong-Le V, Lovalenti P, Abdul-Fattah AM. Stabilization challenges and
formulation strategies associated with oral biologic drug delivery systems.
Adv Drug Deliv Rev. 2015;93:95-108.
48. Klang M. Medication administration in the enetrally fed patient. In:
Duggan CP, Jaksic T, Gura KM, eds. Clinical Management of Intestinal
Failure. Boca Raton, FL: CRC Press; 2012:359-370.

Boullata et al 85
Section 9. Complication Avoidance and
Error Reporting
Background
Complications associated with EN therapy are often prevent-
able with standardized monitoring protocols and vigilant
care. General complications of EN include GI, mechanical,
and metabolic consequences. For a complete review of over-
all enteral complications, refer to Section 4 on tube place-
ment as well as the ASPEN Adult and Pediatric Core
Curricula. Most complications are not of an immediate
safety concern and therefore will remain outside the scope of
this document. The safety-related complications to be dis-
cussed here include refeeding syndrome, pulmonary aspira-
tion, and enteral misconnections. An enteral misconnection
is an inadvertent connection between an enteral feeding sys-
tem and a nonenteral system, such as an intravascular cath-
eter, peritoneal dialysis catheter, tracheostomy, or medical
gas tubing.
1
Beginning in 2015, a new patient access connec-
tor called ENFit was made available on enteral administra-
tion sets, with enteral syringes and EADs to follow. These
connectors will not be interconnectable with other therapy
connectors such as those on intravenous, respiratory, neur-
axial, or limb-cuff pressure devices.
Question 9.1. How can EN-related refeeding syndrome
be prevented?
Practice Recommendations
1. Identify patients at risk for refeeding syndrome prior to
initiation of EN. Risk factors include:
a. Inadequate nutritional intake for >2 weeks
b. Poorly controlled diabetes
c. Cancer, both before and during treatment
d. Anorexia nervosa
e. Short bowel syndrome
f. Inflammatory bowel disease
g. Being an elderly patient living alone
h. Low birth weight and premature birth
i. Chronic infections (eg, HIV)
2. Monitor fluid balance, daily weight, and electrolyte
status (eg, potassium, magnesium, phosphorus), as
well as other metabolic parameters (eg, glucose) as
needed based on the patient’s presenting clinical
situation.
3. Evaluate metabolic and nutrition parameters, and
correct metabolic abnormalities or depleted
electrolyte concentrations prior to the initiation of
enteral feedings.
4. Initiate 25% of goal requirements on day 1 of EN.
5. Provide supplemental thiamin (IV or PO) with EN
initiation.
6. Monitor parameters (serum potassium, phosphorus,
magnesium, glucose) following EN initiation and
replace as needed.
Rationale
Refeeding syndrome is a condition that occurs when malnour-
ished patients are refed near their goal rate.
2–6
It is manifest by
rapid shifts in both intracellular and extracellular electrolytes,
which can cause life-threatening complications (Table 5).
Monitoring of these metabolic parameters prior to the initiation
of enteral feedings and periodically during EN therapy is based
on protocols and the patient’s underlying disease state and length
of therapy. Prevention of refeeding syndrome is of utmost impor-
tance. Patients at high risk for refeeding syndrome and other
metabolic complications must be identified and followed closely,
and depleted minerals and electrolytes should be replaced prior to
initiating nutrition support. Stanga et al
2
highlighted cases of
refeeding syndrome, and each case developed 1 or more features
of refeeding syndrome, including deficiencies and low plasma
concentrations of potassium, phosphate, magnesium, and thiamin
combined with sodium and water retention.
These patients
responded to specific interventions; however, in most cases, these
abnormalities could have been anticipated prior to feeding and
prevented.
Other cases have been described in the literature.
4
EN can be initiated at approximately 25% of the estimated
goal and advanced cautiously over 3–5 days toward the goal
rate. Serum electrolytes, volume status, clinical manifestations,
and vital signs are monitored carefully after EN is started.
3
Question 9.2. How can EN-related pulmo nary aspiration
be prevented?
Practice Recommendations
1. Routinely evaluate all enterally fed patients for risk of
aspiration.
2. Actively employ steps to reduce risk of aspiration.
3. Verify that the feeding tube is in the proper position
before initiating feedings.
4. Keep sedation level as minimal as possible.
5. Insert or advance the feeding tube with tip in the small
bowel for patients with high risk of aspiration.
6. Keep the HOB elevated at 30 to 45 at all times during
the administration of gastric enteral feedings.
7. Deliver EN continuously rather than intermittently in
patients with intolerance to gastric bolus feedings.
8. Consider a course of promotility agents (eg,
metoclopramide or erythromycin) where clinically
feasible in patients with high risk of aspiration.

86 Journal of Parenteral and Enteral Nutrition 41(1)
9. GRV measurements may not need to be used as part of
routine care to monitor ICU patients on EN. For those
patient care areas where GRVs are still utilized, holding
EN for GRVs <500 mL in the absence of other signs of
intolerance should be avoided. A gastric residual
volume of between 250 and 500 mL should lead to
implementation of measures to reduce risk of aspiration
as defined elsewhere in this document.
Rationale
In most patient populations, pulmonary aspiration can cause
chemical pneumonitis and lead to significant complications
such as hypoxia and bacterial pneumonia. Aspiration can be
defined as the inhalation of material into the airway. In the
critically ill patient, this material may include nasopharyngeal
secretions and bacteria as well as liquids, food, and gastric con-
tents.
7
The risk factors for aspiration include sedation, supine
patient positioning, the presence and size of a nasogastric tube,
malposition of the feeding tube, mechanical ventilation, vomit-
ing, bolus feeding delivery methods, the presence of a high-
risk disease or injury, poor oral health, nursing staffing level,
and advanced patient age and patient transfers for procedures
to other units and facilities.
8
Much of the research and many of
the recommendations presented here come from the critical
care literature and may not explicitly be extrapolated to all
patient populations; however, the principles generally apply.
EAD positioning. Proper positioning of the tip of the EAD is
of major importance in the prevention of aspiration. Migration
of the tube to an inappropriate position such as the esophagus
can be a factor in the regurgitation and aspiration of gastric
contents. The American Association of Critical-Care Nursing
prevention of aspiration practice alert recommends that the
position of feeding tubes be checked every 4 hours.
9
Radio-
graphs are the “gold standard” for placement verification, but
it is not practical to do frequent x-rays on most patients simply
to confirm the position of the tip of the tube. Other suggested
methods of checking EAD placement include marking the
point where the feeding tube enters the nares or penetrates the
abdominal wall (in the case of a gastrostomy or jejunostomy
tube) and then assessing whether the mark shifts, or measuring
and documenting the visible tube length. Although this tech-
nique can give some information, it does not verify the position
of the tip of the tube. Methods such as aspiration of GI con-
tents/tube feeds or insufflation and auscultation alone are unre-
liable in determining the position of the tube tip.
9
Endotracheal intubation impairs the swallowing reflex.
Modern, “soft-cuff” endotracheal tubes do not completely
occlude the tracheal lumen and allow for the passage of small
quantities of liquids into the pulmonary system. Also, the
presence of an object in the trachea allows for the partial
compression of the esophagus due to pressure of the object
across the membranous portion of the trachea. Therefore,
although endotracheal intubation may help prevent massive
aspiration from vomiting, it does not prevent aspiration of
small amounts and therefore cannot be considered protective
against aspiration.
Changing the level of infusion of EN from the stomach to
the small bowel has been shown to reduce the incidence of
regurgitation, aspiration, and pneumonia.
10,11
In 13 randomized
controlled trials, pneumonia was significantly lower in patients
with small bowel EN. Compared to patients on gastric EN, the
lower rates were significant even when studies were restricted
to those using evidence of ventilator-associated pneumonia.
However, there were no differences in mortality, ICU length of
stay, hospital length of stay, duration of mechanical ventilation,
or time to goal EN.
12–23
It may be necessary to feed the child at risk for aspiration
into the small bowel. Gastrojejunostomy tubes are recom-
mended for pediatric patients who require long-term EN and
have demonstrated intolerance to gastric feedings due to
delayed gastric emptying, gastroesophageal reflux, or risk of
aspiration.
24
Sedation. Other steps to decrease aspiration risk include reduc-
ing the level of sedation/analgesia when possible and minimiz-
ing transport for diagnostic tests and procedures.
25,26
Any
treatment that impairs the ability of the patient to clear contents
in the pharynx increases the risk of aspiration. Under normal
circumstances, the presence of any material in the pharynx
induces a swallowing or coughing reflex, which helps to pre-
vent aspiration. Sedation of a patient decreases or eliminates
this reflex and increases the risk of aspiration. Keeping patient
comfort and care in mind, it is advisable to keep sedation levels
as minimal as possible to minimize the suppression of the swal-
lowing/coughing reflexes.
Table 5. Nutrient Deficiencies and Potential Complications
Associated With Refeeding Syndrome.
Nutrient Deficiency Manifesting Complication
Phosphorus Cardiac arrhythmia and sudden death
Congestive heart failure
Respiratory failure
Renal failure from osmotic diuresis
Hemolysis
Altered mental status
Potassium Cardiac arrhythmia
Respiratory failure
Paresthesias, paralysis, seizures
Ileus
Rhabdomyolysis
Magnesium Cardiac arrhythmias, sudden death
Respiratory failure
Paresthesias
Paralysis
Seizures, tetany
Thiamin Korsakoff’s syndrome
Wernicke’s encephalopathy

Boullata et al 87
Positioning of patient. One study compared patients in supine
and semirecumbent positions. The investigators found that ele-
vating the head of the bed 30–45 reduced the incidence of
pneumonia from 23% to 5% (P = .018).
27
Infants and children
lying flat are at increased risk of reflux and aspirating formula. It
is important to position the infant or child to prevent aspiration
episodes, with the head of the child elevated at least 30° while
receiving a feeding.
28,29
It is generally recommended that infants
under 1 year of age be positioned on their back in a flat position.
However, the American Academy of Pediatrics guidelines make
exceptions for infants whose risk of death from complications of
gastroesophageal reflux is greater than the risk of sudden infant
death syndrome (SIDS), including those infants with anatomical
abnormalities, such as type 3 or 4 laryngeal clefts, who have not
had antireflux surgery. Otherwise, elevating the head of the bed
while the infant is supine is not recommended.
30
Chlorhexidine mouthwashes. In 2 studies, optimizing oral
health with chlorhexidine mouthwashes twice daily reduced
respiratory infection and nosocomial pneumonia in patients
undergoing heart surgery.
31,32
Studies where chlorhexidine oral
care was included in bundled interventions showed significant
reductions in nosocomial respiratory infections.
33,34
Bolus vs continuous infusions. The potential harm from aggres-
sive bolus infusion of EN leading to increased risk of aspiration
pneumonia was shown in 1 study.
35
A randomized controlled
trial showed a trend toward decreased mortality with continu-
ous EN (13.9% intermittent vs 7.4% continuous, P = .18).
36
Five small randomized controlled trials comparing bolus to
continuous infusion have shown greater volume with fewer
interruptions in delivery of EN with continuous EN, but there
was no significant difference between techniques with regard to
patient outcome.
37–41
Use of prokinetic agents. Oral or intravenous use of prokinetic
agents such as erythromycin or metoclopramide has been
shown to improve gastric emptying and tolerance of EN.
Erythromycin doses of 3–7 mg/kg/d have been used to treat
gastric enteral feeding intolerance. Likewise, metoclopramide
10 mg given 4 times a day has been shown to be efficacious for
elevated gastric residuals; however, dosage adjustments to
metoclopramide may be necessary in patients with declining
renal function. Use of prokinetic agents such as erythromycin
or metoclopramide has resulted in little change in clinical out-
come for ICU patients. A total of 8 randomized controlled trials
using metoclopramide and 1 trial combining erythromycin
with metoclopramide were reviewed by meta-analysis. The use
of prokinetic agents was not found to make a difference in
terms of mortality or infection.
42–49
Erythromycin has been
associated with undesirable effects, including cardiac toxicity,
tachyphylaxis, and bacterial resistance, and should be used
cautiously with monitoring. Metoclopramide also has associ-
ated adverse complications, including tardive dyskinesia, more
frequently in the elderly. Both agents have been associated
with QT prolongation, predisposing to cardiac arrhythmias.
50,51
Combination therapy with erythromycin and metoclopramide
demonstrated improved GRVs allowing for greater feeding
success; however, neither hospital length of stay (LOS) nor
mortality was improved. Furthermore, the incidence of watery
diarrhea was statistically higher in patients receiving combina-
tion therapy.
44
In pediatrics, the risks of the use of metoclo-
pramide should be very carefully considered.
Measurement of GRVs. Measurement of GRV has tradition-
ally been one technique used as an indicator for aspiration risk.
Research regarding the efficacy of this technique has provided
conflicting results. That is, no adequately powered studies
have, to date, demonstrated a relationship between aspiration
pneumonia and GRV.
52
In addition, no adequately powered
studies have demonstrated that elevated GRVs are reliable
markers for increased risk of aspiration pneumonia. Building a
protocol around risk for aspiration could include several fac-
tors to reduce risk but not be solely based on measurement of
G RV.
53
GRV cannot be correlated with pneumonia (after the
initiation of enteral feedings), ICU mortality, or hospital mor-
tality. Studies suggest that “the elevated residual volumes by
themselves have little clinical meaning and that only when
combined with vomiting, sepsis, sedation, or the need for vaso-
pressor agents does the correlation with worsening patient out-
come emerge.”
54
Elevated and increasing residual volumes
may be a symptom of another underlying problem manifesting
itself as delayed gastric emptying. If serial measurements
reveal a change in GRV, other potential causes must be investi-
gated rather than simply holding the enteral feedings.
54
Results
from 4 randomized controlled trials indicate that raising the
cutoff value for GRVs (leading to automatic cessation of EN)
from a lower number of 50–150 mL to a higher number of
250–500 mL does not increase the incidence of regurgitation,
aspiration, or pneumonia.
20,54–56
Decreasing the cutoff value
for GRVs does not protect the patient from these complica-
tions. Use of GRVs leads to increased EAD clogging, inappro-
priate cessation of EN, consumption of nursing time, and
allocation of healthcare resources and may adversely affect
outcome through reduced volume of EN delivered.
57
Note that
GRV measurement may be dependent on type of EAD as well
as patient position.
Three studies (2 randomized controlled trials and 1 pro-
spective before/after implementation trial) have shown that
eliminating the practice of measuring GRVs improves delivery
of EN without jeopardizing patient safety.
57–59
All 3 trials
showed no significant difference between groups with regard
to pneumonia.
57–59
Two of the trials found that elimination of
GRV measurement was associated with significantly greater
EN delivery, either by increased volume of EN infused or
greater reduction in energy deficit.
58,59
If the practice of GRVs is eliminated, a number of alterna-
tive strategies may be used to monitor critically ill patients

88 Journal of Parenteral and Enteral Nutrition 41(1)
receiving EN, including careful daily physical examinations,
review of available abdominal radiologic films, and evaluation
of clinical risk factors for aspiration. Those ICUs that are
reluctant to stop using GRVs are advised to take care in their
interpretation. GRVs in the range of 200–500 mL may raise
concern and lead to the implementation of measures to reduce
risk of aspiration, but it is not appropriate to stop EN for GRVs
<500 mL in the absence of other signs of intolerance.
19,25,54–56
Pediatric considerations. In neonatology, GRV measurement
was once thought to be part of a prevention strategy for necrotiz-
ing enterocolitis.
60
However, because checking gastric residuals
is associated with a high percentage of held feeds and failure to
meet enteral feeding goals without being a good marker of feed-
ing intolerance, some neonatal clinicians no longer check residu-
als.
61
Holding feeds in response to GRVs can be a major reason
why infants do not meet their feeding goals,
62,63
but the clinical
value of GRVs for assessing feeding tolerance in this population
is not established. GRV levels are not considered a marker of
feeding intolerance in premature infants due to their immature
motility. Higher residuals in premature infants are thought to be
related to position (with left lateral and supine positions being
associated with higher volumes), as well as the degree of prema-
turity and normal dysmotility of prematurity.
64–66
Also, by the
time an infant has residuals, he or she may already have necro-
tizing enterocolitis or an ileus from sepsis.
Question 9.3. What are the current methods to prevent
enteral misconnections?
Practice Recommendations
1. Utilize enteral devices (tubes, syringes, administration
and extension sets) with enteral connectors that comply
with ISO standard 80369-3 (ENFit).
2. Review currently used systems to assess practices that
include the potential for misconnection, including
nonstandard, rigged work-arounds (Luer adapters, etc).
3. Train nonclinical staff and visitors not to reconnect
lines but to seek clinical assistance instead. Only
clinicians or users knowledgeable about the use of any
device should make a reconnection.
4. Make connections under proper lighting.
5. Do not modify or adapt IV or feeding devices because
doing so may compromise the safety features
incorporated into their design.
6. When making a reconnection, routinely trace lines
back to their origins and then ensure that they are
secure.
7. When arriving at a new setting or as part of a hand-off
process, recheck connections and trace all tubes.
8. Identify and confirm the EN label. Note that a 3-in-1
PN admixture can appear similar to an EN formulation
bag.
Rationale
Reports of enteral misconnections date as far back as 1972,
when a case of an inadvertent intravenous (IV) administration
of breast milk was published.
67
In 1 literature review, over 115
cases of published enteral misconnections were reported.
68
The
published reports consistently substantiate the highest level of
severity for this type of error, which commonly results in the
death of the patient by embolus or sepsis,
1
but current reporting
may greatly underestimate the number of actual cases or near-
miss incidents involving feeding connectors.
In April 2006, The Joint Commission issued a Sentinel
Event Alert on tubing misconnections. It stated that multiple
reports to agencies such as The Joint Commission, ECRI
Institute, U.S. Food and Drug Administration, Institute for Safe
Medication Practices (ISMP), and USP indicated that these
misconnection errors were occurring with significant frequency
and, in a number of instances, had deadly consequences. The
alert also identified root causes and risk reduction strategies.
69
Despite many other healthcare alerts on medical misconnec-
tions from various safety and regulatory agencies, errors involv-
ing misconnections continued.
1
That Joint Commission alert in
2006 called for a connector design that prevents cross-connec-
tions between IV and enteral products and asserted that any
other remedies might decrease risk but would not eliminate it.
For example, color-coding enteral connectors (for which there
is no current authorized standard color) simply alerts the clini-
cian that the connector is not an IV connector, but a unique
color does not physically prevent the misconnection.
69
In 2008, the International Organization of Standardization
(ISO) convened a working group to develop standards for the
redesign of small-bore connectors. ISO standards are recog-
nized by many national governments, organizations, and other
entities as the resource to drive conformity. As such, ISO sets
voluntary global standards to which various governments, pur-
chasing organizations, manufacturers, and users subscribe.
70
The first step in this process was developing a master standard
for small-bore connectors that contained certain requirements
to which all small-bore connectors must adhere. That standard
is ISO 80369-1: Small Bore Connectors for Liquids and Gases
in Healthcare Applications—Part 1: General Requirements.
71
According to these general requirements, connectors must
Not be connectable to others in the series
Be made of rigid or semi-rigid material
Pass a misconnection test
Not be connectable with Luer or needleless connector
ports
This master standard set the stage for redesigned connectors to
be used in respiratory, enteral, noninvasive blood pressure
monitoring, neuraxial, urology, and intravascular systems.
The first ISO-compliant patient access enteral connector,
called ENFit, can be seen in Figure 11.

Boullata et al 89
This new connector is available on enteral administration
sets, enteral syringes, and enteral feeding tubes. These products
began to be introduced into the market in 2015. To transition
from the new connector to the current feeding tube, a transition
set is available to provide connectivity so that patients receive
their nutrition formula, hydration, and medications.
Communication about these changes is available from the
Global Enteral Device Supply Association (GEDSA), which
has launched a campaign for the introduction of new small-bore
connectors called StayConnected (www.StayConnected.org).
In August 2014, The Joint Commission issued a Sentinel
Event Alert titled Managing Risk During Transition to New ISO
Tubing Connector Standards which included the background on
the issue and a series of actions suggested by The Joint
Commission.
72
These suggested strategies included preparing for
the new standards, development of effective processes and proce-
dures, education and training of staff, effective communication,
and leadership and emphasis on a safety culture. The alert also
included a table of related Joint Commission requirements for
institutions and agencies regarding the use of tubing.
72
Question 9.4. What are the best practices to
systematically identify, document, and report errors
associated with EN within an organization and
externally to patient safety organizations?
Practice Recommendations
1. Develop and provide education within healthcare
organizations for clinicians responsible for the
prescription, dispensing, and administration of EN.
2. Coordinate education with ongoing competency
assessments and should be dynamic to each provider’s
practice setting, institution-specific errors, and changes
in EN guidelines and practice recommendations.
3. Develop and provide education regarding EN and
medical device safety for ancillary staff and healthcare
students (medical, nursing, allied professions).
4. Support mechanisms to systematically report any and
all errors associated with any step in the EN process,
including those related to enteral medication
administration.
5. Create a “culture of safety” within healthcare
organizations where healthcare clinicians will not be
reprimanded for reporting errors related to EN.
6. Develop interdisciplinary teams to evaluate and
analyze errors related to EN within an organization.
7. Provide outpatient EN services with processes for
evaluating patient and caregiver competency related to
EN.
8. Develop and implement policies and procedures to
systematically collect, document, and report errors
associated with EN to the Institute for Safe Medication
Practices (ISMP) Medication Errors Reporting Program.
9. Develop and implement policies and procedures to
systematically collect, document, and report errors
associated with EN infusion pumps and devices to the
U.S. Food and Drug Association (FDA).
Rationale
Safe practices for EN therapy involve a broad interplay of pro-
viders, departments, and administrative support structures
across the many steps of the EN process. Errors can occur from
patient assessment to prescribing, order review, and documen-
tation, although most recognized errors focus on product selec-
tion and administration. Maintaining a safety culture around
EN depends on continuous surveillance, recognition of poten-
tial risk at each step in the process, and systematic reporting of
all errors—including near misses. Monitoring and reporting
safety issues can allow for subsequent system improvements
upon review by an organization’s committee or a national
patient safety organization.
73
The improper administration of EN has led to patient harm
and even death. The use of resources such as patient safety
reporting databases and national patient safety organizations is
vital to identify issues associated with the delivery of EN. As
clinicians report events, national patient safety organizations
and local healthcare organizations can take the proper steps to
analyze events, determine their root causes or weaknesses in the
EN process, and establish changes and new recommendations
to prevent future errors.
Healthcare organizations can use published clinical guide-
lines to develop nursing policies and procedures for EN admin-
istration. Review of these policies and procedures by a
multidisciplinary team on at least an annual basis will identify
issues related to the EN process and can prevent future break-
downs in the process. Healthcare organizations in all settings
Figure 11. ENFit enteral connector. Reprinted with permission
from the Global Enteral Device Supply Association (GEDSA).

90 Journal of Parenteral and Enteral Nutrition 41(1)
can provide continuous education of healthcare providers,
patients, and caregivers for those administering EN.
Competency must be validated at critical times, such as the
following
74
:
During orientation of new medical and ancillary staff
Before a change in organizational policy or procedure
Before a change in equipment, EN products, or
infusion sets
When deficits in EN administration knowledge are
present
The reporting of EN-related errors or close calls is best viewed
as an opportunity to improve patient safety.
73
The underreport-
ing of adverse events related to EN hinders a healthcare orga-
nization’s ability to identify and address gaps in policies and
procedures. For example, few organizations systematically
report and review EAD occlusion rates. Healthcare organiza-
tions can create a culture of safety by reassuring workers that
they will not be punished for reporting safety events and fram-
ing these events as an opportunity for education.
72
The education of ancillary staff and student practitioners
within a healthcare organization is important to the safety of
patients receiving EN. Organizations also need to identify
ancillary staff who could possibly be responsible for the con-
nection, disconnection, or reconnection of devices attached to
patients and develop policies and procedures that outline
responsibilities for these staff members relating to the connec-
tion or disconnection of medical tubing.
74
EN infusion pumps are a necessity in some patients receiv-
ing EN in both the acute and home setting. These pumps are not
error free, and they can malfunction, leading to inappropriate
delivery of EN. Evans et al
75
looked at overnight EN safety
issues in children with metabolic disorders. In this study, 32%
of patients had faulty equipment (leaking EN bags or tubing
defects), and 50% of patients had total pump failure that affected
feeding accuracy, with 1 patient becoming hypoglycemic and
hospitalized. Policies and procedures related to the safe opera-
tion of EN infusion pumps can explain appropriate caregiver
roles regarding alarm silencing, adjusting pump settings, and
making the connection or disconnection from a patient.
76
Healthcare organizations are advised to develop policies
and procedures that address the collection, documentation,
and reporting of errors related to EN to the ISMP’s National
Medication Errors Reporting Program (MERP). After analysis
of adequately reported medication errors, the ISMP has
responded with nationwide hazard alerts to healthcare profes-
sionals with safety issues and error reduction recommenda-
tions. The ISMP has also been able to distribute press releases
regarding safety issues to both the lay and healthcare media.
These reports have led to individual practice and organiza-
tional system changes. Analysis of errors reported to the
MERP has led the ISMP to release guidelines regarding stan-
dardized order sets.
76–78
Organizational policies and procedures related to EN admin-
istration that address EN infusion pump errors or failure are valu-
able. Organizations can use the voluntary MedWatch Form FDA
3500 to report device malfunctions to the FDA. Adequate report-
ing of any medical device issues allows for the FDA to detect
potential device-related safety issues. Organizations are required
to report all device-related deaths or serious injuries to the FDA
and manufacturer within 10 working days of becoming aware of
the event using Form FDA 3500A. User facilities are required to
send an annual summary of deaths and serious injuries to the
FDA with Form FDA 3419 by January 1 for the preceding year.
79
Topics for Future Research
Clinical outcomes from combination promotility
therapy as well as the associated risk of adverse effects
The transition to new enteral connectors in the
marketplace, particularly in neonates and home care
Adverse events reported by the FDA and ISMP as
measures of change effectiveness
Cost-effectiveness of workflow processes to prepare
medications for EAD administration
Identify existing error rates at each step in the EN
process
References
1. Guenter P, Hicks RH, Simmons D, et al. Enteral feeding misconnec-
tions: a consortium position statement. Jt Comm J Qual Patient Saf.
2008;34:285-292.
2. Stanga Z, Brunner A, Leuenberger M, et al. Nutrition in clinical prac-
tice—the refeeding syndrome: illustrative cases and guidelines for preven-
tion and treatment. Eur J Clin Nutr. 2008;62:687-694.
3. Kraft MD, Btaiche IF, Sacks GS. Review of the refeeding syndrome. Nutr
Clin Pract. 2005;20:625-633.
4. Boateng AA, Sriram K, Mequid MM, Crook M. Refeeding syndrome:
treatment considerations based on collective analysis of literature case
reports. Nutrition. 2010;26:156-167.
5. Crook MA, Hally V, Panteli JV. The importance of the refeeding syn-
drome. Nutrition. 2001;17:632-637.
6. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the pro-
vision and assessment of nutrition support therapy in the adult criti-
cally ill patient: SCCM and A.S.P.E.N. JPEN J Parenter Enteral Nutr.
2016;40:159-211.
7. Zaloga GP. Aspiration-related illnesses: definitions and diagnosis. JPEN J
Parenter Enteral Nutr. 2002;26(6)(suppl):S2-S7.
8. Metheny NA. Risk factors for aspiration. JPEN J Parenter Enteral Nutr.
2002;26(6)(suppl):S26-S31.
9. American Association of Critical-Care Nurses. AACN practice alert pre-
vention of aspiration. Crit Care Nurs. 2016:36(1):e20-e24.
10. Heyland DK, Drover JW, MacDonald S, Novak F, Lam M. Effect of
postpyloric feeding on gastroesophageal regurgitation and pulmonary
microaspiration: results of a randomized controlled trial. Crit Care Med.
2001;29(8):1495-1501.
11. Lien HC, Chang CS, Chen GH. Can percutaneous endoscopic jejunos-
tomy prevent gastroesophageal reflux in patients with preexisting esopha-
gitis? Am J Gastroenterol. 2000;95(12):3439-3443.
12. Davies AR, Morrison SS, Bailey MJ, et al. A multicenter, randomized
controlled trial comparing early nasojejunal with nasogastric nutrition in
critical illness. Crit Care Med. 2012;40(8):2342-2348.

Boullata et al 91
13. Acosta-Escribano J, Fernandez-Vivas M, Grau Carmona T, et al.
Gastric versus transpyloric feeding in severe traumatic brain injury: a
prospective, randomized trial. Intensive Care Med. 2010;36(9):1532-
1539.
14. Hsu CW, Sun SF, Lin SL, et al. Duodenal versus gastric feeding in medi-
cal intensive care unit patients: a prospective, randomized, clinical study.
Crit Care Med. 2009;37(6):1866-1872.
15. Kearns PJ, Chin D, Mueller L, Wallace K, Jensen WA, Kirsch CM. The
incidence of ventilator-associated pneumonia and success in nutrient
delivery with gastric versus small intestinal feeding: a randomized clinical
trial. Crit Care Med. 2000;28(6):1742-1746.
16. Montecalvo MA, Steger KA, Farber HW, et al. Nutritional outcome and
pneumonia in critical care patients randomized to gastric versus jeju-
nal tube feedings. The Critical Care Research Team. Crit Care Med.
1992;20(10):1377-1387.
17. Montejo JC, Grau T, Acosta J, et al. Multicenter, prospective, randomized,
single-blind study comparing the efficacy and gastrointestinal complica-
tions of early jejunal feeding with early gastric feeding in critically ill
patients. Crit Care Med. 2002;30(4):796-800.
18. Kortbeek JB, Haigh PI, Doig C. Duodenal versus gastric feeding in ven-
tilated blunt trauma patients: a randomized controlled trial. J Trauma.
1999;46(6):992-998.
19. Taylor SJ, Fettes SB, Jewkes C, Nelson RJ. Prospective, randomized, con-
trolled trial to determine the effect of early enhanced enteral nutrition on
clinical outcome in mechanically ventilated patients suffering head injury.
Crit Care Med. 1999;27(11):2525-2531.
20. Minard G, Kudsk KA, Melton S, Patton JH, Tolley EA. Early versus
delayed feeding with an immune-enhancing diet in patients with severe
head injuries. JPEN J Parenter Enteral Nutr. 2000;24(3):145-149.
21. Day L, Stotts NA, Frankfurt A, et al. Gastric versus duodenal feeding
in patients with neurological disease: a pilot study. J Neurosci Nurs.
2001;33(3):148-149, 155-159.
22. Davies AR, Froomes PR, French CJ, et al. Randomized comparison of
nasojejunal and nasogastric feeding in critically ill patients. Crit Care
Med. 2002;30(3):586-590.
23. White H, Sosnowski K, Tran K, Reeves A, Jones M. A randomized con-
trolled comparison of early post-pyloric versus early gastric feeding to
meet nutritional targets in ventilated intensive care patients. Crit Care.
2009;13(6):R187.
24. Egnell C, Eksborg S, Grahnquist L. Jejunostomy enteral feed-
ing in children: outcome and safety. JPEN J Parenter Enteral Nutr.
2013;38(5):631-636.
25. McClave SA, DeMeo MT, DeLegge MH, et al. North American summit
on aspiration in the critically ill patient: consensus statement. JPEN J
Parenter Enteral Nutr. 2002;26(6)(suppl):S80-S85.
26. Kollef MH. Prevention of hospital-associated pneumonia and ventilator-
associated pneumonia. Crit Care Med. 2004;32(6):1396-1405.
27. Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer
M. Supine body position as a risk factor for nosocomial pneumo-
nia in mechanically ventilated patients: a randomized trial. Lancet.
1999;354(9193):1851-1858.
28. Baker SS, Baker RD, Davis AM. Pediatric Nutrition Support. Sudbury,
MA: Jones and Bartlett; 2007.
29. Bankhead R, Boullata J, Brantley S, et al; A.S.P.E.N. Board of Directors.
Enteral nutrition practice recommendations. JPEN J Parenter Enteral
Nutr. 2009;33:122-167.
30. Task Force on Sudden Infant Death Syndrome; The American Academy
of Pediatrics. SIDS and other sleep related infants deaths: expansion of
recommendations for a safe infant sleeping environment. Pediatrics.
2011;128:1030-1039.
31. DeRiso AJ II, Ladowski JS, Dillon TA, Justice JW, Peterson AC.
Chlorhexidine gluconate 0.12% oral rinse reduces the incidence
of total nosocomial respiratory infection and nonprophylactic sys-
temic antibiotic use in patients undergoing heart surgery. Chest.
1996;109(6):1556-1561.
32. Houston S, Hougland P, Anderson JJ, LaRocco M, Kennedy V, Gentry
LO. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reduc-
ing prevalence of nosocomial pneumonia in patients undergoing heart sur-
gery. Am J Crit Care. 2002;11(6):567-570.
33. Simmons-Trau D, Cenek P, Counterman J, Hockenbury D, Litwiller L.
Reducing VAP with 6 sigma. Nurs Manage. 2004;35(6):41-45.
34. Zack JE, Garrison T, Trovillion E, et al. Effect of an education program
aimed at reducing the occurrence of ventilator-associated pneumonia. Crit
Care Med. 2002;30(11):2407-2412.
35. Ibrahim EH, Mehringer L, Prentice D, et al. Early versus late enteral feed-
ing of mechanically ventilated patients: results of a clinical trial. JPEN J
Parenter Enteral Nutr. 2002;26(3):174-181.
36. MacLeod JB, Lefton J, Houghton D, et al. Prospective randomized control
trial of intermittent versus continuous gastric feeds for critically ill trauma
patients. J Trauma. 2007;63(1):57-61.
37. Bonten MJ, Gaillard CA, van der Hulst R, et al. Intermittent enteral
feeding: the influence on respiratory and digestive tract colonization in
mechanically ventilated intensive-care-unit patients. Am J Respir Crit
Care Med. 1996;154(2, pt 1):394-399.
38. Steevens EC, Lipscomb AF, Poole GV, Sacks GS. Comparison of con-
tinuous vs intermittent nasogastric enteral feeding in trauma patients: per-
ceptions and practice. Nutr Clin Pract. 2002;17(2):118-122.
39. Hiebert JM, Brown A, Anderson RG, Halfacre S, Rodeheaver GT, Edlich
RF. Comparison of continuous vs intermittent tube feedings in adult burn
patients. JPEN J Parenter Enteral Nutr. 1981;5(1):73-75.
40. Kocan MJ, Hickisch SM. A comparison of continuous and intermittent
enteral nutrition in NICU patients. J Neurosci Nurs. 1986;18(6):333-337.
41. Ciocon JO, Galindo-Ciocon DJ, Tiessen C, Galindo D. Continuous com-
pared with intermittent tube feeding in the elderly. JPEN J Parenter
Enteral Nutr. 1992;16(6):525-528.
42. Berne JD, Norwood SH, McAuley CE, et al. Erythromycin reduces
delayed gastric emptying in critically ill trauma patients: a randomized,
controlled trial. J Trauma. 2002;53(3):422-425.
43. Chapman MJ, Fraser RJ, Kluger MT, Buist MD, De Nichilo DJ.
Erythromycin improves gastric emptying in critically ill patients intoler-
ant of nasogastric feeding. Crit Care Med. 2000;28(7):2334-2337.
44. Meissner W, Dohrn B, Reinhart K. Enteral naloxone reduces gastric tube
reflux and frequency of pneumonia in critical care patients during opioid
analgesia. Crit Care Med. 2003;31(3):776-780.
45. Nguyen NQ, Chapman M, Fraser RJ, Bryant LK, Burgstad C, Holloway
RH. Prokinetic therapy for feed intolerance in critical illness: one drug or
two? Crit Care Med. 2007;35(11):2561-2567.
46. Nursal TZ, Erdogan B, Noyan T, Cekinmez M, Atalay B, Bilgin N. The
effect of metoclopramide on gastric emptying in traumatic brain injury. J
Clin Neurosci. 2007;14(4):344-348.
47. Yavagal DR, Karnad DR, Oak JL. Metoclopramide for preventing pneu-
monia in critically ill patients receiving enteral tube feeding: a randomized
controlled trial. Crit Care Med. 2000;28(5):1408-1411.
48. Reignier J, Bensaid S, Perrin-Gachadoat D, Burdin M, Boiteau R,
Tenaillon A. Erythromycin and early enteral nutrition in mechanically
ventilated patients. Crit Care Med. 2002;30(6):1237-1241.
49. MacLaren R, Kiser TH, Fish DN, Wischmeyer PE. Erythromycin vs
metoclopramide for facilitating gastric emptying and tolerance to intra-
gastric nutrition in critically ill patients. JPEN J Parenter Enteral Nutr.
2008;32(4):412-419.
50. Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians
should know about the QT interval. JAMA. 2003;289(16):2120-2127.
51. Li EC, Esterly JS, Pohl S, Scott SD, McBride BF. Drug-induced
QT-interval prolongation: considerations for clinicians. Pharmacotherapy.
2010;30(7):684-701.
52. Kattelmann KK, Hise M, Russell M, Charney P, Stokes M, Compher
C. Preliminary evidence for a medical nutrition therapy protocol: enteral
feedings for critically ill patients. J Am Diet Assoc. 2006;106: 1226-1241.
53. Metheny NA, Davis-Jackson J, Stewart BJ. Effectiveness of an aspiration
risk-reduction protocol. Nurs Res. 2010;59(1):18-25.

92 Journal of Parenteral and Enteral Nutrition 41(1)
54. McClave SA, Lukan JE, Stefater JA, et al. Poor validity of residual vol-
ume as a marker for risk of aspiration in critically ill patients. Crit Care
Med. 2005;33:324-330.
55. Montejo JC, Minambres E, Bordeje L, et al. Gastric residual volume dur-
ing enteral nutrition in ICU patients: the REGANE study. Intensive Care
Med. 2010;36(8):1386-1393.
56. Pinilla JC, Samphire J, Arnold C, Liu L, Thiessen B. Comparison of
gastrointestinal tolerance to two enteral feeding protocols in critically
ill patients: a prospective, randomized controlled trial. JPEN J Parenter
Enteral Nutr. 2001;25(2):81-86.
57. Powell KS, Marcuard SP, Farrior ES, Gallagher ML. Aspirating gastric
residuals causes occlusion of small-bore feeding tubes. JPEN J Parenter
Enteral Nutr. 1993;17(3):243-246.
58. Reignier J, Mercier E, Le Gouge A, et al. Effect of not monitoring residual
gastric volume on risk of ventilator-associated pneumonia in adults receiv-
ing mechanical ventilation and early enteral feeding: a randomized con-
trolled trial. JAMA. 2013;309(3):249-256.
59. Poulard F, Dimet J, Martin-Lefevre L, et al. Impact of not measuring
residual gastric volume in mechanically ventilated patients receiving early
enteral feeding: a prospective before-after study. JPEN J Parenter Enteral
Nutr. 2010;34(2):125-130.
60. Cobb BA, Carlo WA, Ambalavanan N, et al. Gastric residuals and their
relationship to necrotizing enterocolitis in very low birth weight infants. J
Pediatr Gastroenterol Nutr. 2004;113:50-53.
61. Parker L, Torrazza RM, Li Y, et al. Aspiration and evaluation of gas-
tric residuals in the NICU: state of the science. J Perinat Neonatal Nurs.
2014;29:51-59.
62. Torazza RM, Parker LA, Li Y, Talaga E, Shuster J, Neu J
. The value of
routine evaluation of gastric residuals in very low birth weight infants. J
Perinatol. 2015;35(1):57-60.
63. Li YF, Lin HC, Torazza PM et al. Gastric residual evaluation in preterm
neonates: a useful monitoring tool or a hindrance? Pediatr Neonatol.
2014;55:335-340.
64. Shulman RJ, Ou CN, Smh EO. Evaluation of potential factors predict-
ing attainment of full gavage feedings in preterm infants. Neonatology.
2011;99:38-44.
65. Sangers Hm de Jong PM, Mulder SE, et al. Outcomes of gastric residuals
whilst feeding preterm infants in various body positions. J Neonatal Nurs.
2013;19:337-341.
66. Cohen S, Mardel D, Mimount FB, et al. Gastric residual in growing
preterm infants: effect of body position. Am J Perinatol. 2004;21: 163-
166.
67. Wallace JR, Payne RW, Mack AJ. Inadvertent intravenous breast milk.
Lancet. 1972;1(7763):1264-1266.
68. Simmons D, Symes L, Guenter P, Graves K. Tubing misconnections: nor-
malization of deviance. Nutr Clin Pract. 2011;26:286-293.
69. Joint Commission Sentinel Event Alert. Tubing misconnections—a per-
sistent and potentially deadly occurrence. April 3, 2006. http://www.joint-
commission.org/SentinelEvents/SentinelEventAlert/sea_36.htm. Accessed
April 23, 2015.
70. Guenter P. New enteral connectors: raising awareness. Nutr Clin Pract.
2014;29(5):612-614.
71. ISO Small Bore Connectors Working Group. ANSI/AAMI/ISO 80369-
1:2010. Small Bore Connectors for Liquids and Gases in Healthcare
Applications—Part 1: General Requirements. Arlington, VA: Association
for the Advancement of Medical Instrumentation; 2011.
72. Joint Commission Sentinel Event Alert. Managing risk during transition to
new ISO tubing connector standards. August 20, 2014. http://www.joint-
commission.org/sea_issue_53/
. Accessed April 23, 2015.
73. Boullata JI. Safe practices for enteral and parenteral nutrition. In: Seres
DS, Van Way CW, eds. Nutrition Support for the Critically Ill. New York,
NY: Springer; 2016:229-241.
74. Adhikari R, Tocher J, Smith P, Corcoran J, MacArthur J. A multi-dis-
ciplinary approach to medication safety and the implication for nursing
education and practice. Nurse Educ Today. 2014;34:185-190.
75. Evans S, MacDonald, Daily A, Hopkins V, Holden C. Home enteral tube
feeding in patients with inherited metabolic disorders: safety issues. J
Hum Nutr Diet. 2007;20(5):440-445.
76. Institute for Safe Medication Practices. ISMP’s guidelines for standard
order sets. http://www.ismp.org/tools/guidelines/StandardOrderSets.asp
.
Accessed July 20, 2015.
77. Vaida AJ, Lamis RL, Smetzer JL, Kenward K, Cohen MR. Assessing the
state of safe medication practices using the ISMP Medication Safety Self
Assessment of Hospitals: 2000 and 2011. Jt Comm J Qual Patient Saf.
2014;40(2):51-67.
78. Grissinger M. Guidelines for Standard Order Sets. P T. 2014;39(1): 10-11, 50.
79. U.S. Food and Drug Administration. Medwatch voluntary report. https://
www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.
home
. Accessed July 15, 2015.
Section 10. Monitoring and Reassessment
Background
The goal of nutrition reassessment is to update the nutrition
care plan based on changes in clinical or nutrition status as
identified through ongoing monitoring and evaluation.
Monitoring the patient receiving EN can help prevent iatro-
genic malnutrition and other adverse events. The timeframe for
follow-up of enterally fed patients is often driven by an organi-
zation’s policy and procedures.
Making certain that the patient receives the amount of for-
mula recommended and ordered is important to optimize
nutrition status and prevent malnutrition. Volume delivered is
often not the amount ordered. In most cases, volume delivered
is less than what was ordered, with the most common reasons
being GI intolerance, such as nausea, vomiting, abdominal
distention, or diarrhea; tube obstruction or dislodgement; or
feeding interruptions for nursing or physician care, proce-
dures, or patient refusal. Measuring the volume the patient
received within a specified timeframe is important to deter-
mine whether nutrient needs are being met. Methods to track
volume delivered are not standardized and may include
recording intake and output, documenting number of hours
the feeding was held vs infusion hours ordered, marking the
bottle or bag, or logging the volume measured by the enteral
feeding pump, with the latter method possibly allowing the
least room for human error. Reassessment will also include
the transition from EN to oral nutrition as appropriate.
Question 10.1. What are the minimum monitoring
parameters and timeframes for reassessment to allow
for safe management of the patient receiving EN?
Practice Recommendations
1. Monitor and evaluate the patient receiving EN to
identify all changes in physical examination findings,
laboratory values, anthropometric data, and outcomes.
2. Include a thorough review of changes in clinical status,
new medications and therapies, EN intake and
tolerance, biochemical indices, anthropometric

Boullata et al 93
changes (eg, physical examination and weight), and
malnutrition risk.
3. Assess nutrition risk of the patient receiving EN
throughout the patient’s therapy.
a. Determine frequency of assessment by considering
patient acuity and progression of clinical care.
b. Provide regular documentation of patient
reassessment—typically, daily and/or weekly.
Monitoring of nutrition status may be more
frequent than documentation of reassessment.
4. Reassess the tube-fed patient in institutionalized long-
term care at least monthly.
5. Reassess the home tube-fed patient at least quarterly.
Rationale
Reassessment timeframes will depend on the practice setting.
EN intolerance will likely be noted within the first 3 days of
initiation, if at all.
1
Monitoring parameters focus on changes in
clinical status that will likely affect tolerance of the enteral pre-
scription. Tolerance is measured using various methodologies,
which are discussed elsewhere in this document. It is important
to monitor the adverse effects of medications or particular
forms of medications that can affect the safety of EN. For
example, liquid medications with high sorbitol content may
contribute to loose stools, dehydration, and perceived intoler-
ance of the enteral formula. Interruptions to EN, including
those due to intolerance of EN, are monitored because they can
contribute to undernutrition.
2
Energy deficit is associated with
increased clinical complications, especially infections.
3
It is
important to monitor that medications are administered sepa-
rately and diluted appropriately to prevent clogging of the tube
and missed nutrition.
Routine laboratory monitoring will assist the clinician in
determining overall tolerance of the nutrition treatment plan.
4
The nutrition assessment and recommendations section found
earlier in this document addresses this factor in more detail.
Unintentional weight loss is a risk factor on validated malnu-
trition screening tools and therefore must be monitored
closely in all patients on EN. Last, organizations need proto-
cols to monitor and prevent potential adverse effects associ-
ated with EN, such as aspiration. The clinician can monitor
oral hygiene and the use of other precautions, such as elevat-
ing the HOB to at least 30 to 45 during and after tube
feeding.
Question 10.2. How is EN tolerance best determined?
Practice Recommendations
1. Assess tolerance to EN using a combination of
parameters appropriate to the individual patient.
2. Evaluate patient subjective complaints, objective
findings of GI function (eg, GRV, vomiting, diarrhea),
and physical examination findings (eg, abdominal
distension).
Rationale
Patients in all settings and age groups must be monitored while
undergoing EN support. Monitoring EN tolerance is essential in
the delivery of EN because patients who experience EN intoler-
ance frequently fail to achieve EN goals.
5
Monitoring for EN
intolerance often includes multiple parameters such as GRV
and assessment of GI function.
6
In a recent observational study,
Wang and colleagues
7
reported that 32% of patients receiving
EN in a large tertiary hospital experienced enteral feeding intol-
erance. Of those patients, approximately two-thirds demon-
strated a single high GRV, whereas one-third experienced a
combination or 2 or more of the following symptoms: high
GRV, nausea/vomiting, and abdominal distention. Blaser and
colleagues
5
recently evaluated EN intolerance in ICU patients
with the objective to identify a definition most strongly associ-
ated with ICU mortality. They concluded the “best” definition
of EN intolerance is based on “a complex assessment of GI
symptoms” rather than a single measurement.
GRV monitoring and interventions to improve EN tolerance
based on this assessment are covered elsewhere in this docu-
ment. The American Society for Parenteral and Enteral
Nutrition/Society of Critical Care Medicine nutrition guide-
lines, recommendation D1 states that “patients should be moni-
tored daily for tolerance of EN (determined by physical exam,
passage of flatus or stool, radiologic evaluations and absence of
patient complaints such as pain or abdominal distention).”
Inappropriate cessation of EN should be avoided.
8
Question 10.3. What is the best way to transition from
EN to oral feeding?
Practice Recommendations
1. Identify a safe oral feeding regimen through discussion
with interdisciplinary team members, including speech
and language specialists who evaluate swallowing and
aspiration risk with various food consistencies. Provide
an individualized diet with necessary modifications in
the initial stages of oral intake.
2. Transition continuous EN to an intermittent
schedule when clinically appropriate. Provision of
either partial or full EN via this intermittent regimen
will depend on the nutrition needs and status of the
patient.
3. Coordinate oral feedings with times when EN is off
to help stimulate appetite. Consider intermittent EN
feedings that are administered as a supplement after
a meal is consumed and/or continuous feedings at
night.
4. Establish a consistent meal routine.

94 Journal of Parenteral and Enteral Nutrition 41(1)
5. Document the percentage of food consumed at each
meal or snack. Ideally, the type and amount of food
are also recorded.
6. Document any identified issues with oral consumption.
7. Involve the patient and/or family members in food
and oral supplement preferences regarding oral diet
advancement.
8. Monitor swallowing performance, nutrition and
hydration status, and respiratory complications with
adjustment of EN as appropriate.
9. Consider a trial of eliminating the EN regimen when
the patient is able to meet the majority of energy needs
with oral intake.
10. Obtain weights at least weekly to ensure adequate
caloric intake to meet weight goals.
Rationale
Transitional orders from EN to oral feeding have been defined
as incremental decreases in EN volume over a period of time
to accommodate for increasing oral intake. Minimal research
or guidelines exist regarding transition from enteral feeding to
oral feeding. Crary and Groher
9
recommends that at mini-
mum, tube-fed patients with dysphagia must demonstrate a
safe and efficient swallow on a consistent basis to be consid-
ered candidates to return to oral feeding. Additionally, patients
must be able to consume adequate food or liquid to support
nutrition requirements before they can be fully transitioned
from EN to oral feeding.
Buchholz
10
developed a clinical algorithm specific to
patients with acquired brain injury or stroke that provides sug-
gestions for transitioning tube-fed patients to oral feeding. The
initial transition phase is termed the preparatory phase and
focuses on the patient’s physiologic readiness for oral nutri-
tion. This first phase incorporates medical and nutrition stabil-
ity, intermittent tube-feeding implementation, and swallowing
assessment. The second phase is termed weaning and includes
a graduated increase in oral feeding, with corresponding
decreases in tube feeding. In this algorithm, once a patient is
consuming 75% or more of his or her nutrition requirements
consistently by mouth for 3 days, all tube feedings are discon-
tinued. Another proposed option is nighttime cycling of EN
once patients are meeting more than 60% of target calories by
the oral route.
11
Clinical reality dictates that both patients and healthcare
professionals will vary in terms of their readiness to discon-
tinue enteral feeding. The process of transition should be
thoroughly discussed with the patients, assuming that they
are clinically able to communicate, and outline a plan of
action. Simple and patient-specific goals are often helpful.
Oral ingestion is best attempted at times when the stomach is
not full, taking full advantage of the hunger drive. Continuous
feedings can be modified to an intermittent schedule to stim-
ulate normal hunger cycles, and ideally, intermittent
feedings are tolerated before an oral diet is attempted. When
patients attempt oral feedings, it is important that they are
fully upright and alert. For patients with fluctuating mental
status, try feeding when their pattern of alertness is maximal.
Therefore, for these patients, return to oral intake may only
involve attempts at 1 or 2 meals per day. If patients have
swallowing difficulties, a speech-language pathologist can
recommend appropriate types and textures of food to put on
trays.
During the transition process, it is important to remember
that the total time to wean from tube feeding to oral feeding is
patient dependent. Also, weaning from tube to oral nutrition is
not a goal shared by all patients.
12
Abrupt discontinuation of
nutrition therapy predisposes the patient to hypoglycemia if an
insulin regimen is not adjusted. A reduction in the nutrition
support infusion rate without an adjustment in insulin therapy
was the second most common cause for hypoglycemia in 1 ret-
rospective study.
13
Close monitoring of glycemic control in
patients transitioning from enteral to oral diet is critical to pre-
vent sentinel events.
Topics for Future Research
Optimal frequency of nutrition screening/reassessment
based on outcomes
Optimal protocol to transition EN to order feeding
Updated data on pump accuracy studies using pumps
currently in use in the United States
References
1. Gungabissoon U, Hacquoil K, Baines C, et al. Prevalence, risk factors,
clinical consequences, and treatment of enteral feed intolerance during
critical illness. JPEN J Parenter Enteral Nutr. 2015;39(4):441-448.
2. Elpern EH, Stutz L, Peterson S, Gurka DP, Skipper A. Outcomes associ-
ated with enteral tube feedings in a medical intensive care unit. Am J Crit
Care. 2004;13(3):221-227.
3. Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocalo-
ric feeding and energy balance on clinical outcome in ICU patients. Clin
Nutr. 2005;24(4):502-509.
4. Mueller CM, Compher C, Druyan ME. A.S.P.E.N. clinical guidelines:
nutrition screening, assessment, and intervention in adults. JPEN J
Parenter Enteral Nutr. 2011;35(1):16-24.
5. Blaser AR, Starkopf L, Deane AM, Poeze M, Starkopf J. Comparison of
different definitions of feeding intolerance: a retrospective observational
study. Clin Nutr. 2015;34(5):956-961.
6. Malone AM, Seres DS, Lord L. Challenges and complications with
enteral nutrition. In: Mueller CM, ed. The Science and Practice of
Nutrition Support: A Case Based Curriculum. 3rd ed. Silver Spring,
MD: American Society for Enteral and Parenteral Nutrition; 2012:218-
233.
7. Wang K, McIiroy K, Plank LD, et al. Prevalence, outcomes and man-
agement of enteral tube feeding intolerance: a retrospect cohort study in
a tertiary center [published online February 5, 2016]. JPEN J Parenter
Enter Nutr.
8. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provi-
sion and assessment of nutrition support therapy in the adult critically ill
patient: Society of Critical Care Medicine (SCCM) and American Society
for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter
Enteral Nutr. 2016;40:159-211.

Boullata et al 95
9. Crary MA, Groher ME. Reinstituting oral feeding in tube-fed patients with
dysphagia. Nutr Clin Pract. 2006;21(6):576-586.
10. Buchholz AC. Weaning patients with dysphagia from tube feeding to oral
nutrition: a proposed algorithm. Can J Diet Pract Res. 1998;59:208-214.
11. Collier BR, Cherry-Bukowiec JR, Mills ME. Trauma, surgery, and
burns. In: Mueller CM, ed. The A.S.P.E.N. Adult Nutrition Support Core
Curriculum. 2nd ed. Silver Spring, MD: ASPEN:392-411.
12. Corrigan ML, Escuro AA, Celestin J, Kirby DF. Nutrition in the stroke
patient. Nutr Clin Pract. 2011;26(3):242-252.
13. Dickerson RN, Maish GO, Minard G, Brown RO. Nutrition support team-
led glycemic control program for critically ill patients. Nutr Clin Pract.
2014;29(4):534-541.
Section 11. Transition of Care
Background
A major goal of transitioning the EN patient to home or an alter-
nate care site is to prevent readmission to the acute facility.
Therefore, many factors need to be considered to ensure that
patients have everything for the safe and successful admission of
EN at home or in another setting. Institutions play a critical role
in ensuring the safe transition and adequate education of patients
and caregivers to home EN therapy. Optimal transition to home
EN requires a collaborative approach among all disciplines and
professions involved in the care of the patient.
1,2
Disciplines
involved in the transition of home EN patients can use guide-
lines, policies, and procedures to best serve the interests and
safety of these patients. It is advisable to commence the educa-
tion of the patient and caregiver as early as possible so potential
problems and concerns can be identified.
3
Question 11.1. What are the criteria and factors to
consider to safely transition a patient on EN from the
hospital to home or an alternate care site?
Practice Recommendations
1. Establish tolerance to EN at the goal regimen prior to
discharge.
2. Provide written and verbal instruction to the patient
and/or caregivers well before discharge.
3. Ascertain that the patient/caregiver demonstrates
competence in all components of the EN therapy.
4. Assess safety of the home environment by including
home care provider and case manager in the process.
5. Utilize competent nutrition clinicians to monitor home
EN therapy.
6. Prior to discharge, educate the patient/caregiver on
how to obtain necessary supplies.
Rationale
To ensure a safe transition of the EN patient from the hospital
to home setting, multiple conditions must be met. Most impor-
tant, the patient must tolerate the EN therapy (formula, rate,
and volume) to be continued in the home or alternate care set-
ting. Patients experiencing EN intolerance are at much greater
risk of developing complications that may require hospital
readmission, including tube blockage, GI issues, and underhy-
dration or overhydration. The patient/caregivers will need edu-
cation about the EN therapy to be used in the new setting.
Written and verbal instruction can begin well before discharge
and will include the following: all elements of the EN prescrip-
tion, including water-flushing regimen and treatment plan;
care of the feeding tube; troubleshooting of common complica-
tions; and who to contact for help at any time. Instruction to
specifically address water requirements and flushing is impor-
tant. In a study of older adults receiving EN at home, 73% of
patients reported decreased urination and 63% reported consti-
pation.
1
As part of the instruction process, educators must eval-
uate the patient/caregiver’s ability to demonstrate competence
in the fundamental aspects of EN therapy.
Prior to discharge, the home environment must be screened
for safety. A clean water supply, refrigeration and electricity, a
sanitary environment, sufficient space to administer feedings and
store supplies, and telephone access are required to safely admin-
ister EN at home.
2
In addition, the home must have resources
available for use during emergencies. For alternate site care, the
care team at the hospital thoroughly discusses the patient’s plan
of care with the receiving facility’s nutrition support expert.
The lack of professional nutrition services in the home setting
may increase the risk of preventable complications. In 1 report,
one-third of older adult patients receiving EN at home reported
tube clogs or leaks, problems that increase the risk for underfeed-
ing, dehydration, or stoma complication. Almost one-third of
patients using an EN pump reported pump malfunction, which
increases the risk of underhydration and underfeeding.
1
The need
for tube changes is another common complication of EN.
3
These
patients need to be managed and monitored by healthcare profes-
sionals who are competent in nutrition support and are available
to respond to complications. In addition, collaboration and com-
munication between these professionals are essential.
2,4
Patients receiving EN at home often feel isolated as a result
of their therapy.
5
To help prevent such isolation, clinicians
involved in preparing patients for EN at home can refer them
to a support organization, such as the Oley Foundation or the
Feeding Tube Awareness Foundation, which can help patients
when the need for EN at home is established and prior to hos-
pital discharge.
5
When preparing patients for home, the care
team can also establish an initial and ongoing process for
obtaining all necessary supplies, including initial review and
verification of insurance and/or third-party coverage prior to
discharge. In addition, the home medical supply company or
alternate site must be able to supply formula, equipment, and
supplies prior to or upon discharge. It is certainly advanta-
geous to the patient’s safety and comfort if the home supply
company provides competent nutrition clinicians to address
education needs, tolerance, complications, and nutrition ade-
quacy of EN at home.

96 Journal of Parenteral and Enteral Nutrition 41(1)
Questions 11.2–11.4. What components of EN education
are important to promoting efficacy, safety, and
quality of life for the caregiver/patient? What are the
most effective methods of caregiver/patient EN
education/instruction considering literacy and
safety? When should education/instruction for
patients receiving EN at home be performed?
Practice Recommendations
1. Begin the referral process once the decision for EN
therapy is made.
2. Begin education for the patient receiving EN at home
prior to placement of the EAD.
3. Provide patient and caregiver education that is
comprehensive, includes education materials related to
EN therapy, and uses a standard checklist.
4. Provide the patient and caregiver with verbal and
written education that covers the following topics:
a. Reason for EN and short-term and long-term
nutrition goals (ie, weight goal)
b. Feeding device, route and method, formula, and
feeding regimen
c. Identify necessary supplies needed to administer
enteral tube feedings at home
d. Use and cleaning of equipment, including
administration/feeding set, infusion pump, and
syringe
e. Care of the feeding tube and access site such as
securing, flushing, and unclogging the tube and
stoma care
f. Nutrition and hydration guidelines: feeding plan/
regimen, water flushes, hydration monitoring
g. Weight schedule, lab work recommendations
h. Safe preparation and administration of formula
i. Safe preparation and administration of medications
j. Proper position during and after feedings
k. Recognition and management of complications
(mechanical, gastrointestinal, and metabolic)
l. Available resources, emergency care plan, and
healthcare contacts
5. Use demonstration and teach-back method of patient
education to assess comprehension.
6. Use various methods of education for EN to take into
account various learning styles.
7. Implement an EN education checklist to assist with the
discharge coordination process.
Rationale
Effective patient and caregiver education is an integral part of
discharge for patients going home on EN and can start soon
after the decision is made to transition a patient. Inadequate ini-
tial EN education and follow-up have been reported as
challenges associated with EN at home.
6
A study of parents of
children receiving EN at home reported that most parents indi-
cated a need for improved EN services. Parents wanted a more
structured follow-up and would have preferred that 1 healthcare
professional coordinate EN education and discharge.
7
Institutions need guidelines, protocols, and policies for the
safe provision of EN to adult and pediatric patients as well as
procedures for ensuring a safe discharge to home on EN. Home
care and supply companies should continue this process after
discharge. When possible, it is advisable to provide training for
more than 1 person on all aspects of tube care and feeding
management. Essential components of the education process
include training on feeding tube and access site care, prepara-
tion and administration of formula, medication administration,
enteral pump operation, monitoring and troubleshooting com-
plications, and emergency care plan and contact informa-
tion.
6,8–12
Thompson et al
13
have emphasized the need for
clinicians to evaluate the effectiveness of their EN education
process, provide comprehensive EN education and patient
resources, proceed over more than 1 educational session, and
prepare patients and caregivers to resolve foreseeable prob-
lems, such as tube occlusion and dislodgement, skin care
issues, and psychosocial challenges.
Patients receiving EN at home may cope more effectively
and comply more successfully with the EN plan when clinicians
actively seek their input regarding the feeding plan and craft a
plan that is as flexible as possible to conform with the family’s
lifestyle. Flexibility within feeding regimens may alleviate some
of the stress that patients have and has the potential to improve
the impact of EN on quality of life. Simplifying the EN regimen,
minimizing the infusion time, and providing an ambulatory
pump or feeding tube that best fits the patient’s physical and life-
style needs may help reduce EN-associated life disruptions.
13
For some patients with gastrostomy tubes, transition to home
may be made easier by employing the syringe/bolus feeding
method. Feedings are ideally scheduled to fit as conveniently as
possible with the patient’s home and/or work routine. In addi-
tion, the clinician can help the patient/caregiver understand why
changes to the enteral or medication regimen could result in
adverse outcomes.
6
Another opportunity to help patients cope
with the day-to-day living on home nutrition support is to refer
the patient to a support group or organization specific to the dis-
ease or therapy the patient is experiencing.
Various methods may be used to deliver education for the
patient receiving EN at home. The most effective methods for
a given patient or caregiver will take into account the individ-
ual’s specific learning styles and provide visual demonstration
and reinforcement with graphics, video, online tutorials, nutri-
tion education handouts, or other approaches that best suit the
learner. Language and health literacy are factors to consider in
the instruction process. Clayton
14
authored an invited review to
aid selection of effective patient nutrition education materials
and has identified some key features of the healthcare delivery
system that may detract from the effectiveness of EN education

Boullata et al 97
and negatively affect the patient’s ability to safely administer EN
at home, including decreased patient-provider contact time,
length of hospital stay, and increased patient responsibility for
self-care. When selecting education materials, it is important to
evaluate them for content, literacy level, graphics, layout, and
typography. The motivating principles, cultural relevance and
primary language, feasibility (cost, equipment needs), and
accessibility are other factors to consider in patient education.
When online sources are used, educators need to evaluate the
references’ credibility and help patients find reliable Internet
resources. For example, websites can be reviewed for potential
conflicts of interest, disclaimers, and disclosures, and the ease of
navigation and interactivity can also be evaluated.
14
An EN discharge checklist helps the educator and patient/
caregiver document and track stepwise instruction. Use of a
discharge checklist has been shown to enhance patient care
and help streamline the discharge coordination process.
15
The
Agency for Clinical Innovation and the Gastroenterological
Nurses College of Australia clinician’s guide provides an
example of an EN checklist.
16
Items detailed in this example
include tube/device and site care, the nutrition and hydration
plan, regimen details and preparation instructions, proce-
dures for supply procurement and refills, monitoring, follow-
up care, and contact details. The checklist can also document
the date(s) that instruction was given and whether the patient
and/or caregiver can demonstrate the instructions, as indi-
cated by patient/caregiver and educator signatures.
16
Optimal EN education begins before the EAD is placed.
Preoperative education may increase the patient’s comfort
level, allay anxiety, reduce the hospital length of stay, and
improve patient satisfaction. Whenever possible, the patient
and family must be made aware before the procedure of poten-
tial complications and scope of care of the feeding tube, as well
as what costs of care will be reimbursed.
17
Identifying con-
cerns early can help alleviate some of the patient’s fears and
potential misconceptions about having a feeding tube. Early
educational interventions also provide opportunities to assess
the ability of the patient to care or obtain care for the tube and
administer feedings.
Question 11.5. What is the best method to communicate
enteral prescriptions and care instructions during
patient transfer or discharge home or alternate care
site?
Practice Recommendations
1. Determine the safest and most effective mechanism for
communicating the EN care plan. See Figure 12.
2. Involve representatives of the discharging site
(nutrition support clinician, case manager, or
prescriber) and the accepting site or home care team
(nutrition support clinician, home supply company,
home health agency) in planning the care transition.
3. Transfer the EN prescription and regimen to the
accepting home care team (nutrition support clinician/
home supply company/home health agency) via
standard electronic information systems accessible to
all healthcare providers and suppliers associated with
the patient prior to discharge.
4. Communicate the EN regimen to the home care team
caring for the patient.
Rationale
Adequate and timely transfer of information between inpatient
and community settings is imperative for safe care of EN
patients.
18
Incomplete or incorrect communication of the EN pre-
scription and regimen during patient transfer may delay the
administration of adequate and appropriate nutrition. It may also
lead to hospital readmissions and emergency department visits
that may have been preventable.
1
See Figure 12 for a template of
information that should be available for safe transitions. Ideally,
the enteral prescription and regimen are transferred to the accept-
ing home care team via standard electronic information systems
that are accessible to all healthcare providers and suppliers associ-
ated with the patient.
19
Use of these systems may improve com-
munication; however, they may not be universally available or
accessible due to technical limitations or institutional policies. The
EN prescription and regimen are best communicated in the avail-
able medical record. Effective communication of the EN plan is
written in plain language, includes all essential elements, and does
not use abbreviations that might lead to misinterpretation and
error. Ideally, the EN plan is provided to the home health agency
or medical supply company prior to discharge.
1,19
If a change or
clarification of the EN prescription must be communicated by
phone, the person receiving the new information should repeat it
back to ensure that it is received and interpreted correctly.
Clear and complete communication of the EN prescription will
cover the feeding method, the name of the formula and any modu-
lar additives, the calorie concentration, the rate in milliliters per
hour if pump fed, and the volume of formula per feeding or per
day, as well as the duration of the feeding—for example, [full
name of specific formula product, including concentration] at 75
mL/h times 22 hours to provide 1650 mL daily. Clear and com-
plete instructions about water flushes are also part of the EN pre-
scription and regimen communicated to the patient or caregiver
and the home care team.
1
An example of these instructions would
be as follows: 250 mL of water 4 times daily plus 50 mL water
before, with, and after each medication. If feeding tubes are
included in the EN prescription, instructions about the brand, type,
French size, and length, if applicable, are also communicated.
16
Communication of the home EN regimen, including guidance on
tube replacement and medication administration, is relevant to all
healthcare providers and suppliers involved in the patient’s care.
An interdisciplinary team consisting of the case manager, pre-
scriber, nurse, dietitian, and homecare provider can facilitate the
effective communication of the nutrition prescription.
20,21

98 Journal of Parenteral and Enteral Nutrition 41(1)
CURRENT EN ORDER
Patient Name: ______________________ Medical Record Number: ________________
Date of Birth: _______________ Current Dosing Weight: __________ kg Height _____________ cm
Anticipated Discharge/Transfer Date: __________________ To: _______________________________
EN FORMULA
Energy _____________ kcal/d Protein ________________ g/d Carbohydrate _________________ g/d Fat _____________ g/d
[] Standard [] Fiber Containing [] Elemental or Peptide-based
[] Standard, High Protein [] Carbohydrate Controlled [] Immune-modulating
[] Standard, High Calorie [] Renal, Low Electrolyte
DELIVERY SITE
(Route and Access)
ROUTE: ACCESS:
[] Gastric [] Nasogastric [] Orogastric [] Gastrostomy
[] Small Bowel [] Nasoduodenal [] Oroduodenal [] Jejunostomy
[] Nasojejunal [] Orojeujunal [] Transgastric G/J tube
ADMINISTRATION
(Method and Rate)
Method: Rate:
[] Continuous [] Currently at ______ mL/h with goal of _____ mL/h
[] Intermittent [] Currently ______ mL feeding over _____ mins _____ times daily
With goal of ______ mL feeding over _____ mins _____ times daily
[] Bolus [] Currently ______ mL bolus over _____ mins _____ times daily
With goal of ______ mL bolus over _____ mins _____ times daily
Water Flush _______ mL every _____ hours (minimum volume _____ mL)
CURRENT CLINICAL DATA
Current Nutrition Assessment:
History …
Vitals including anthropometrics …
Pertinent findings
Nutritionally focused physical exam
EN tolerance
Tests
Recent lab data
Anticipated Nutrition Care Plan:
Maintain … with goal of ….
Transition to oral diet …
Transition to PN …
Figure 12. Enteral nutrition transition template. EN, enteral nutrition; G/J, gastrojejunostomy; PN, parenteral nutrition.

Boullata et al 99
Question 11.6. If the patient is going home on a different
formula (or different feeding method) than the one used
in the hospital, is it advisable to try it first in the hospital?
Practice Recommendations
1. Use the type of formula that will be administered at
home for a trial period prior to discharge.
2. Use the feeding method that will be administered at
home for a trial period prior to discharge.
3. Avoid making last-minute changes to either the
formula or method just prior to discharge.
Rationale
The hospital provides a safe setting in which patients are
closely monitored for tolerance to EN. However, the patient
may go home on a different enteral formula or feeding method
due to patient preference, lifestyle or ability, insurance cover-
age, or product availability through the home health agency or
the supply company. In this case, the new formula or feeding
method should be used in the hospital setting for a trial period
to avoid potential complications related to intolerance.
21
A trial
may be more important when the patient is to transition from a
more specialized formula to a standard or less-specialized
product or to bolus or gravity feeding from continuous pump
feeding. For example, a patient transitioning from a peptide-
based to a standard formula may be at risk for intolerance and
GI complications that may be more safely handled in the hos-
pital setting. GI complications are common with EN at home
and have the effect of reducing the amount of nutrition deliv-
ered to the patient, increasing the risk of malnutrition.
1
Adequate instruction of the patient or caregiver on the feed-
ing method and formula to be used at home can optimize safety
and adherence to the treatment plan. Ideally, the method or
regimen chosen for home care is one that fits well with the
patient or family’s ability and lifestyle.
8
Planning for discharge
throughout the patient’s hospital stay can reduce the risk of
readmission.
22
Inpatient administration of the EN to be used at
home and education on the EN regimen for home are part of
the planning process.
Topics for Future Research
Patient and caregiver ability, attitudes, and experiences
Effect of insurance coverage for EN at home on
outcomes
Evaluation of EN patients’ experience regarding the
discharge process at home
Evaluate support systems and potential interventions
for caregivers of tube-fed children
Appropriate mechanism for follow-up and monitoring
for patients receiving EN at home
EN and EHRs
Ideal feeding method and formula for EN at home
References
1. Silver HJ, Wellman NS, Arnold DJ, Livingstone AS, Byers PM. Older adults
receiving home enteral nutrition: enteral regimen, provider involvement, and
health care outcomes. JPEN J Parenter Enteral Nutr. 2004;28(2): 92-98.
2. Durfee SM, Adams SC, Arthur E, et al. A.S.P.E.N. standards for
nutrition support: home and alternate site care. Nutr Clin Pract.
2014;29(4):542-555.
3. Planas M, Lecha M, García Luna PP, et al. National registry of home
enteral nutrition in 2003. Nutr Hosp. 2006;21(1):71-74.
4. Klek S, Hermanowicz A, Dziwiszek G, et al. Home enteral nutrition
reduces complications, length of stay and health care costs: results from a
multicenter study. Am J Clin Nutr. 2014;100:609-615.
5. Chopy K, Winkler M, Schwartz-Barcott D, Melanson K, Greene G. A
qualitative study of the perceived value of membership in The Oley
Foundation by home parenteral and enteral nutrition consumers. JPEN J
Parenter Enteral Nutr. 2015;39(4):426-433.
6. Edwards S, Davis AM, Ernst L, et al. Caring for tube-fed children: a
review of management, tube weaning, and emotional considerations.
JPEN J Parenter Enteral Nutr. 2015;39(8):899-909.
7. Shortall C, Aherne M, Boland S, Sheane R, Ward F, Hensey O. Hospital
to home pediatric enteral nutrition—parents need support. Irish Med J.
2015;108(2):46-48.
8. Best C, Hitchings H. Enteral tube feeding—from hospital to home. Br J
Nurs. 2010;19(3):174-179.
9. Best C. Supporting home enteral tube feeding: some considerations.
Nutrition. 2012;17(suppl 7):S6-S10.
10. Best C, Hitchings H. Day case gastrostomy placement for patients in the
community. Br J Nurs. 2010;15(6):272-278.
11. DiBaise JK, Scolapio JS. Home parenteral and enteral nutrition.
Gastroenterol Clin North Am. 2007;36(1):123-144.
12. Majka AJ, Wang Z, Schmitz KR, et al. Care coordination to enhance man-
agement of long-term enteral tube feeding: a systematic review and meta-
analysis. JPEN J Parenter Enteral Nutr. 2014;38(1):40-52.
13. Thompson CW, Durrant L, Barusch A, Olson L. Fostering coping skills
and resilience in home enteral nutrition (HEN) consumers. Nutr Clin
Pract. 2006;21(6):557-565.
14. Clayton LH. Strategies for selecting effective patient nutrition education
materials. Nutr Clin Pract. 2010;25(5):436-442.
15. Arthur E, Greaves, J. The use of an enteral nutrition discharge checklist
to enhance patient care and help streamline the discharge coordination
process. Clinical Nutrition Week 2014. http://pen.sagepub.com/content/
suppl/2013/12/27/38.1.124.DC1/CNW14_All_Abstracts_file_1.
16. Agency for Clinical Innovation and the Gastroenterological Nurses College
of Australia. A Clinician’s Guide: Caring for People With Gastrostomy Tubes
and Devices. Chatswood, Australia: Agency for Clinical Innovation; 2014.
17. Centers for Medicare and Medicaid Services. National Coverage
Determination (NCD) for enteral and parenteral nutrition therapy (180.2).
Centers for Medicare and Medicaid Services website. https://www.cms.
gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=242&
ncdver=1&DocID=180.2&SearchType=Advanced&bc=IAAAABAAA
AAA&. Published July 11, 1984. Accessed October 3, 2016.
18. Bjuresäter K, Larsson M, Nordström G, Athlin E. Cooperation in the care
for patients with home enteral tube feeding throughout the care trajectory:
nurses’ perspectives. J Clin Nurs. 2008;17:3021-3029.
19. Hesselink G, Zegers M, Vernooij-Dassen M, et al. Improving patient dis-
charge and reducing hospital readmissions by using intervention mapping.
BMC Health Serv Res. 2014;14:389.
20. Norma JL, Crill CM. Optimizing the transition to home parenteral nutri-
tion in pediatric patients. Nutr Clin Pract. 2011;26(3):273-285.
21. National Collaborating Centre for Acute Care. Nutrition Support for
Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral
Nutrition. London, UK: National Collaborating Centre for Acute Care;
2006. NICE Clinical Guidelines No. 32.
22. Li J, Young R, Williams MV. Optimizing transitions of care to reduce
hospitalizations. Cleveland Clin J Med. 2014;81(5):312-320.

100 Journal of Parenteral and Enteral Nutrition 41(1)
Section 12. Documentation and Quality
Review Issues
Background
The EN process is highly complex, involving a multistep con-
tinuous process, including patient assessment and EN recom-
mendations, prescribing, order review, the selection/procurement
of enteral products, their preparation and labeling, and EN
administration and monitoring/reassessment. Documentation
throughout the EN process is important and provides a source for
process evaluation from which to identify gaps in process and
outcomes. For example, documentation of the nutrition assess-
ment is core to the process and has a direct impact on patient care.
Question 12.1. What documentation needs to occur at
each step in the EN process?
Practice Recommendations
1. Document nutrient requirements, including energy,
protein, and fluid, in the medical record within 48
hours of admission.
2. Document data used for nutrition assessment, including
nutrient/fluid intake, anthropometric data, weight
changes and goal weight, lab work, functional and
physical assessment, and any other assessment tools
employed. If any data are extrapolated from another
clinician’s note, such as the physical examination,
include from where the information was obtained.
3. Document the EN prescription and ancillary orders
using the EHR as appropriate.
4. Document how the recommended EN regimen meets the
estimated energy, protein, and fluid requirements initially
and any time a different EN regimen is recommended.
5. Provide an EN prescription review mechanism for all
clinicians involved.
6. Document the formula selection and preparation
through policies and procedures and specifically for
each patient in the EHR.
7. Develop and implement EN protocols to improve EN
administration in patients.
Rationale
The first step of documentation in the EN process is determina-
tion of energy requirements to guide the nutrition plan of care.
Wakeham and colleagues
1
performed a chart review in a cohort
of pediatric ICUs and found that patients with documented calo-
rie requirement were more likely to receive EN support than
those without on each of the first 4 days of admission. Patients
with documented calorie requirements had higher total daily
energy intake by the enteral route and by the enteral and paren-
teral route combined. The authors concluded that documentation
of calorie requirement in the medical record within 48 hours of
admission is significantly associated with higher total daily
energy intake and more frequent use of the enteral route for nutri-
tion. Importantly, in this study, the registered dietitian entered
almost all of the calorie requirements that were present early in
medical records. Documented protocols can also affect the qual-
ity of EN care. Kim and colleagues
2
performed a literature review
to identify major barriers to adequate EN intake in critically ill
adults. They found that interruption of EN is often due to avoid-
able causes such as routine nursing procedures and bedside care.
Also, after an interruption occurs, EN may be restarted at a low
rate. They suggest that standardized feeding protocols to prevent
unnecessary cessation of feedings and restart of EN after inter-
ruptions may maximize EN delivery in the ICU.
Question 12.2. What organizational systems/
administrative structures need to be in place to
support a safe EN process?
Practice Recommendations
1. Provide leadership and oversight at the healthcare
organizational level by competent clinicians
knowledgeable in the EN process.
2. Develop and implement policies and evidence-based
practice guidelines to support the individuals involved
in the assessment and care of patients receiving EN.
3. Develop and implement policies and guidelines
collaboratively among all disciplines involved in the
EN process, and align policies and procedures from
various disciplines, departments, and settings within
the organization.
4. Create a formal committee or structure that includes
expert clinicians from all disciplines to provide
oversight of the EN process.
Rationale
Documentation needs to be supported by a strong infrastruc-
ture of organizational systems and administrative oversight.
The EN process involves many disciplines and departments.
An EN process that minimizes risks requires interdisciplinary
collaboration, standardization through guidelines, and practice
alignment among professions, departments, and settings.
Evidence-based practice guidelines targeted at the clinical,
departmental, and organizational levels support a safe EN pro-
cess. Ideally, policies and guidelines addressing nutrition care,
nursing care, and physician prescribing are developed to target
each discipline’s role in the EN process. These guidelines need
to be aligned and complementary to avoid inconsistencies.
Recent literature supports the use of enteral feeding practice
guidelines and feeding algorithms to improve the safety and
efficacy of enteral feedings. Gentles and colleagues
3
found that
introduction of an enteral feeding practice guideline and par-
ticipation by a dietitian in multidisciplinary bedside rounds

Boullata et al 101
improved provision of nutrition support and overall energy
intake. Similarly, Geukers and colleagues
4
demonstrated that
the introduction of a nurse-driven, early EN algorithm and
implementation of a nutrition support team safely and effec-
tively increased the nutrition intake of critically ill children
during the first few days of an ICU stay.
Organizations can use a governing body or committee com-
posed of a multidisciplinary group of content experts, such as a
nutrition committee, to support safe EN practice. This group can
be charged with reviewing and approving guidelines and identi-
fying educational programs and strategies to disseminate evi-
dence-based guidelines and practices. This interdisciplinary
group can also evaluate and respond to changes in the EN pro-
cess, process failures, and data and outcome measures to con-
tinually improve the process to ensure safety and effectiveness.
Question 12.3. What is the role of clinical decision
support in the EN order and review process?
Practice Recommendations
1. Use clinical decision support tools in guiding safe EN
prescribing.
2. Develop and implement procedures for the EN order
review process.
Rationale
The EHR provides the opportunity to use computerized clini-
cal decision support (CDS) to guide accurate prescribing. CDS
involves the use of alerts, algorithms, and rule-based recom-
mendations to guide ordering. The impact of CDS is controver-
sial. Shojania and colleagues
5
conducted a review of studies
that evaluated the effect of computer reminders on processes or
outcomes of care. Their goal was to determine the degree to
which computer reminders changed provider behavior. They
found that computer reminders delivered to physicians during
routine electronic ordering achieved only small to modest
improvement in care, with a median improvement of 4.2%.
The authors concluded that these changes fall below thresholds
that would be considered clinically significant and “constitute
an expensive exercise in trial and error.” Schedlbauer et al
6
performed a systematic review of alerts and other reminders
and prompts to evaluate the impact on prescribing behavior.
They evaluated 27 different types of alerts and prompts and
found that 23 of 27 resulted in a significant improvement in
prescribing behavior and/or reduction in medication errors,
and many of the alerts and prompts were clinically relevant.
The authors concluded that most of the studies that evaluated
the impact of computerized CDS systems show positive and
significant effects. Although these studies specifically target
medication prescribing, the EN process parallels the medica-
tion management process and therefore the study findings are
relevant to EN.
Question 12.4. What organizational quality control
processes need to be implemented for EN safety?
Practice Recommendations
1. Develop and implement enteral feeding algorithms to
improve the provision of nutrition and possibly reduce
length of stay and mortality.
2. Develop organizational guidelines that address safe
enteral practices collaboratively by a multidisciplinary
team.
3. Disseminate the organizational guidelines by
interactive communication/education methods
utilizing individuals with nutrition expertise.
4. Monitor the EN process for safety and effectiveness.
5. Promote active involvement by members of the
nutrition service in the development of electronic EN
orders and clinical documentation to optimize safe and
effective electronic communication.
Rationale
In a multicenter, cluster-randomized trial, Martin and col-
leagues
7
demonstrated that the implementation of evidence-
based algorithms for nutrition support improved the provision
of nutrition support, reduced hospital length of stay, and may
decrease hospital mortality in critically ill patients in both
community and teaching hospitals. Along with initiation of
nutrition support algorithms, other strategies were used to
improve the effectiveness of nutrition support care, including
educational sessions, educational outreach, and audit with
feedback. Guidelines alone are not adequate; they must be sup-
ported by professional collaboration, education, and effective
communication strategies. In a review, Marshall and col-
leagues
8
identified factors that influence nursing nutrition
practice around EN and how these factors contribute to varia-
tions in practice. Evidence-based guidelines were found to be
important, but EN guidelines were often lacking strong recom-
mendations and evidence related to nursing-specific practice,
which limited their usefulness. To increase use of guidelines
and effectively apply these standards to clinical care, the
authors recommend that the implementation of guidelines be
combined with contributions from resource personnel who
have nutrition and clinical expertise. They emphasize that if
the intent is to use guidelines to standardize and improve prac-
tice, the information is best delivered using strong communica-
tion strategies that incorporate social interaction as a component
of this knowledge transfer. The authors also support an inter-
disciplinary, collaborative approach where professionals from
different disciplines (namely, dietitians, nurses, and physi-
cians) function in a supportive organizational environment that
includes integrated and cohesive care and symmetrical power.
This multidisciplinary team can collaborate in nutrition-related
practice, education, and research.

102 Journal of Parenteral and Enteral Nutrition 41(1)
Standardization of EN orders in the EHR is another avenue
for supporting a safe EN process. Since the passage of the
Health Information Technology for Economic and Clinical
Health (HITECH) Act in 2009, hospitals have been imple-
menting EHR at increasing rates. Successful implementation
of an EHR requires input from the clinicians who will use the
EHR to provide patient care regarding how the EHR can be
built and implemented to maximize patient care and avoid
harm to patients. The safety and efficacy of nutrition and nutri-
tion support content in EHR were the focus of a study that
surveyed members of ASPEN. This survey indicated that most
respondents (85.9%) were using an EHR, with the most com-
mon duration of use between 5 and 10 years. The results dem-
onstrated a significant need for improvement in the safety and
effectiveness of the nutrition and nutrition support content of
the EHRs, with an overall rating of fair for this content (ratings
ranged from unacceptable to excellent). The authors conclude
that nutrition support content needs improvement and that
nutrition support clinicians need to be actively involved in con-
tent development and optimization.
9
Question 12.5. What competencies need to be maintained
by clinicians involved in the EN process?
Practice Recommendations
1. Use discipline-specific standards and available
competencies from professional organizations to create
job descriptions for all clinicians involved in the EN
process.
2. Encourage nutrition support clinicians involved in the
EN process to be board certified by one of the
accredited certifying organizations.
3. Develop at the healthcare organizational level
competency evaluations that measure EN core
elements and knowledge for all clinicians involved in
the EN process.
Rationale
Given the complexity and scope of the EN process, each
organization needs an oversight structure, which may reside
within a standing committee. This group is uniquely qualified
to oversee the EN process. In addition, all clinicians involved
in the EN process must be competent and receive ongoing
education/training to ensure safe and effective care. Education
and competencies set by nutrition-related professional orga-
nizations are also important. For example, the standards of
practice (SOP) and standards of professional performance
(SOPP) for registered dietitian nutritionists (RDNs) in nutri-
tion support have been developed by the American Society
for Parenteral and Enteral Nutrition and the Academy of
Nutrition and Dietetics.
10
These standards outline the compe-
tencies needed for dietitians to provide nutrition support care,
including EN. Similar standards are available for other clini-
cians involved in the EN process.
11–13
Board certification in
nutrition support is highly desirable for those involved in the
EN process. For example, the National Board of Nutrition
Support Certification (NBNSC) certification examination
validates that clinicians (dietitians, nurses, pharmacists, phy-
sicians, and physician assistants) have attained the threshold
of skills and knowledge necessary to provide quality nutrition
support care. Additional board certification processes are
available for some of these healthcare professionals. Surveys
of nutrition support professionals indicate that board certifi-
cation is critical to providing safe and effective care to
patients.
14
Brody and colleagues
15
conducted a survey of
healthcare professionals affiliated with ASPEN and used a
case-based scenario based on established clinical guidelines
to evaluate knowledge of nutrition support practices. More
than half of the respondents were board certified by NBNSC,
and the results indicated that those holding the certification
were significantly more likely to choose correct answers
compared to those without the credential. Although a certifi-
cation examination cannot guarantee patient safety, it can
help ensure patient safety by identifying those individuals
who can demonstrate knowledge through a standardized vali-
dated board certification process.
15
Question 12.6. What essential EN administration and
monitoring components should be documented by
nursing staff and at what interval should EN clinical
documentation occur?
Practice Recommendations
1. Document interruptions to enteral feedings, including
reason and length of interruption; this is best done by
the nursing staff.
2. Document HOB elevation, date/time of administration
start and tubing changes, and residuals for gastric
feedings at each shift.
3. Document amount, type, frequency, and rate of
feeding; patient’s response to tube feeding; abdominal
assessment; patency of the tube; condition of the skin
at tube site if placed in abdominal wall; amount of any
additional water; flush volume, frequency, and rate;
and patient and family education.
4. Record intake and output, weights, and methods used
to verify placement of an EAD.
5. Complete the nursing documentation of EN at each
shift or with any change in condition or order.
Rationale
Documentation of nursing care related to EN administration
and monitoring is critical to a safe EN process and can be
supported by protocols and evidence-based guidelines.
2

Boullata et al 103
HOB elevation, the time/date of EN administration, and
residuals are common nursing documentation standards.
According to Mosby’s Nursing Skills,
16
the following docu-
mentation is also recommended: amount, type, frequency,
and rate of feeding; patient’s response to tube feeding;
abdominal assessment; patency of tube; condition of the skin
at tube site if placed in abdominal wall; amount of any addi-
tional water; flush volume, frequency, and rate; and patient
and family education.
Topics for Future Research
How well does documentation at each step of the EN
process identify opportunities for safety improvement
Data on clinical decision support systems and EN
prescribing and safety
References
1. Wakeham M, Christensen M, Manzi J, et al. Registered dietitians mak-
ing a difference: early medical record documentation of estimated
energy requirements in critically ill children is associated with higher
daily energy intake and with use of the enteral route. J Acad Nutr Diet.
2013;13(10):1311-1316.
2. Kim H, Stotts NA, Froelicher ES, Engler MM, Porter C. Why patients
in critical care do not receive adequate enteral nutrition? A review of the
literature. J Crit Care. 2012;27:702-713.
3. Gentles E, Mara J, Diamantidi K, et al. Delivery of enteral nutrition after the
introduction of practice guidelines and participation of dietitians in pediatric
critical care clinical teams. J Acad Nutr Diet. 2014;114(12):1974-1980.
4. Geukers V, Neef M, Dijsselhof M, Sauerwein H, Bos A. Effect of a nurse-
driven feeding algorithm and the institution of a nutritional support team
on energy and macronutrient intake in critically ill children. e-SPEN J.
2012;7(1):e35-e40.
5. Shojania KG, Jennings A, Mayhew A, Ramsay C, Eccles M, Grimshaw
J. Effect of point-of-care computer reminders on physician behavior: a
systematic review. CMAJ. 2010;182(5):216-225.
6. Schedlbauer A, Prasad V, Mulvaney C, et al. What evidence supports the
use of computerized alerts and prompts to improve clinicians’ prescribing
behavior? JAMA. 2009;16(4):531-538.
7. Martin C, Doig G, Heyland D, Morrison T, Sibbald W. Multicentre,
cluster-randomized clinical trial of algorithms for critical-care enteral and
parenteral therapy (ACCEPT). CMAJ. 2004;170(2):197-204.
8. Marshall AP, Cahill NE, Gramlich L, MacDonald G, Alberda C, Heyland
DK. Optimizing nutrition in intensive care units: empowering critical
care nurses to be effective agents of change. AJCC. 2012;21(3):186-194.
9. Vanek V. Providing nutrition support in the electronic health record era:
the good, the bad and the ugly. Nutr Clin Pract. 2012;27:718-737.
10. Brantley SL, Russell MK, Mogensen KM, et al. American Society for
Parenteral and Enteral Nutrition and Academy of Nutrition and Dietetics
revised 2014 standards of practice and standards of professional perfor-
mance for registered dietitian nutritionists (competent, proficient, and
expert) in nutrition support. Nutr Clin Pract. 2014;29:792-828.
11. Mascarenhas MR, August DA, DeLegge MH, et al. Standards of prac-
tice for nutrition support physicians. Nutr Clin Pract. 2012;27(2):
295-299.
12. Tucker A, Ybarra J, Bingham A, et al. A.S.P.E.N. standards of practice for
nutrition support pharmacists. Nutr Clin Pract. 2015;30:139-146.
13. DiMaria-Ghalili RA, Gilbert K, Lord L, et al. Standards of nutrition care
practice and professional performance for nutrition support and generalist
nurses. Nutr Clin Pract. 2016;31(4):527-547.
14. Materese LE, Chinn RN, Hertz NR, Callahan P, Harvey-Banchik L,
Strang B. Practice analysis of nutrition support professionals: evidence-
based multidisciplinary nutrition support certification examination. JPEN
J Parenter Enteral Nutr. 2012;36(6):663-670.
15. Brody R, Hise M, Fleisch Marcus A, Harvey-Banchik L, Matarese L.
Evaluating evidence-based nutrition support practice among healthcare
professionals with and without the certified nutrition support clinician cre-
dential. JPEN J Parenter Enteral Nutr. 2016;40(1):107-114.
16. Mosby’s Skills. Enteral nutrition via nasoenteric, gastrostomy, or jejunos-
tomy tube. http://mns.elsevierperformancemanager.com/NursingSkills
.
Accessed June 1, 2015.
Conclusion
The EN process consists of numerous steps involving several
disciplines that perform a number of specific tasks at each
step. These daily responsibilities are critical to ensuring safe
care of the patient requiring EN therapy. Given the potential
risk for error in the systems within which EN is used, ongoing
systematic surveillance, critical process and outcome evalua-
tion, and quality improvements will support patient safety.
Organizations can incorporate into their system of care the
best practice recommendations within this document, to sup-
port a culture of safety, by applying an interdisciplinary
approach in an accommodating administrative structure.
