
Ammonia:
zero-carbon fertiliser,
fuel and energy store
POLICY BRIEFING

Cover image © 2020 KBR Inc. All Rights Reserved.
Ammonia: zero-carbon fertiliser,
fuel and energy store
Issued: February 2020 DES5711
ISBN: 978-1-78252-448-9
© The Royal Society
The text of this work is licensed under the terms
of the Creative Commons Attribution License
which permits unrestricted use, provided the
original author and source are credited.
The license is available at:
creativecommons.org/licenses/by/4.0
Images are not covered by this license.
This report can be viewed online at:
royalsociety.org/green-ammonia
Policy briefing
Politics and science frequently move on vastly
different timescales. A policymaker seeking
evidence on a new policy will often need the
answer in weeks or months, while it takes
years to design and undertake the research to
rigorously address a new policy question. The
value of an extended investigation into a topic
cannot be understated, but when this is not
possible good evidence is better than none.
The Royal Society’s series of policy briefings
is a new mechanism aiming to bridge that
divide. Drawing on the expertise of Fellows
of the Royal Society and the wider scientific
community, these policy briefings provide
rapid and authoritative syntheses of current
evidence. These briefings lay out the current
state of knowledge and the questions that
remain to be answered around a policy
question often defined alongside a partner.

CONTE NTS
Contents
Executive summary 4
Introduction 6
Current ammonia storage and transport infrastructure 8
Ammonia: health and environmental considerations 10
1. The decarbonisation of ammonia production 12
1.1 Current ammonia production process – brown ammonia 12
1.2 Blue ammonia production – using blue hydrogen from steam methane
reforming (SMR) with carbon capture and storage (CCS) 14
1.3 Green ammonia production – using green hydrogen from water electrolysis 14
1.3.1 Research opportunities 16
1.4 Novel methods for green ammonia synthesis 19
2. New zero-carbon uses for green ammonia 21
2.1 The storage and transportation of sustainable energy 22
2.2 Ammonia for the transportation and provision of hydrogen 26
2.3 Technological opportunities for ammonia as a transport fuel 28
2.4 The use of ammonia in heating and cooling 32
2.5 Energy conversion efficiency 32
3. International perspectives: activities and future opportunities 34
3.1 Japan 34
3.2 Australia 35
3.3 China 35
Conclusions 36
Annex A: Definitions 37
Annex B: Acknowledgements 38
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 3

EXECUTIVE SUMMARY
Executive summary
Future zero-carbon energy scenarios are
predicated on wind and solar energy taking
prominent roles. Matching demand-driven
energy provision with low-carbon energy
security, from these intermittent sources,
requires long-term sustainable energy storage.
This briefing considers the opportunities and
challenges associated with the manufacture
and future use of zero-carbon ammonia, which
is referred to in this report as green ammonia.
The production of green ammonia has the
capability to impact the transition towards
zero-carbon through the decarbonisation of
its current major use in fertiliser production.
Perhaps as significantly, it has the following
potential uses:
• As a medium to store and transport
chemical energy, with the energy being
released either by directly reacting with air
or by the full or partial decomposition of
ammonia to release hydrogen.
• As a transport fuel, by direct combustion in
an engine or through chemical reaction with
oxygen in the air in a fuel cell to produce
electricity to power a motor.
• To store thermal energy through the
absorption of water and through phase
changes between material states (for
example liquid to gas).
With its relatively high energy density of
around 3 kWh/litre and existing global
transportation and storage infrastructure,
ammonia could form the basis of a new,
integrated worldwide renewable energy
storage and distribution solution. These
features suggest ammonia could readily be
a competitive option for transporting zero-
carbon energy by road, rail, ship or pipeline.
Ammonia has been used as a fertiliser for
over a century and has been of fundamental
importance in providing sufficient food to feed
our planet. Current ammonia manufacture
is predominantly achieved through steam
reforming of methane to produce hydrogen
which is fed into ammonia synthesis via the
Haber Bosch process. Ammonia production
currently accounts for around 1.8% of global
carbon dioxide emissions.
Decarbonisation options mainly target the
production of hydrogen either by integrating
carbon capture and storage or through the
production of hydrogen via water electrolysis
using sustainable electricity.
Ammonia use does present challenges.
Human alteration of the global nitrogen cycle,
mainly through the application of ammonia-
based fertilisers, is a contributor to global
declines in biodiversity, widespread air quality
problems and greenhouse gas emissions
across the world. New uses of ammonia, in
the storage, transportation and utilisation
of renewable energy, must therefore be
decoupled from environmental impact, with
particular emphasis on avoiding and effectively
eliminating emissions of nitrogen oxides and
ammonia release.
Finding affordable and effective solutions to
all these challenges, demonstrating technical
feasibility, developing the appropriate
regulations and implementing safety
procedures will be vital to open up more
flexible routes on a global scale towards a
low-carbon energy future.
Over the coming decades, ammonia has the
potential to make a significant impact through
enabling the transition away from our global
dependence on fossil fuels and contributing,
in substantial part, to the reduction of
greenhouse gas emissions.
The production of
green ammonia
has the capability
to impact the
transition towards
zero-carbon.
4 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

EXECUTIVE SUMMARY
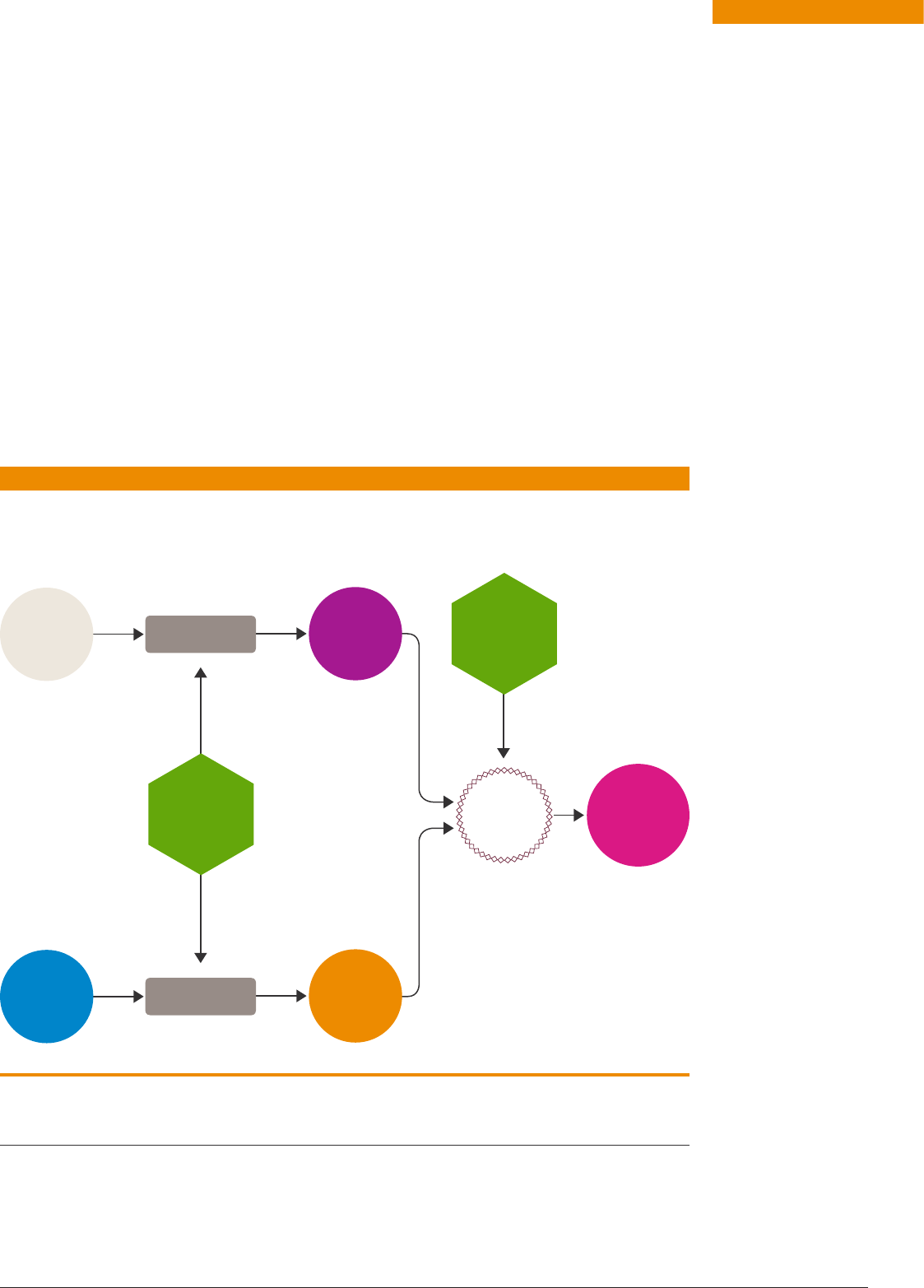
FIGURE 1
Water Air
Electrolysis
Separation
Sustainable
electricity
HABER-
BOSCH
PROCESS
Sustainable
electricity
Hydrogen
Nitrogen
EXISTING USES
Fertilisers
Refrigeration
Explosives
Textiles and
pharmaceuticals
EXPANDED USES
(after cracking)
in PEM fuel cell
Using
alkaline
fuel cell
Direct
combustion
engine/turbine
Energy store to electricity generation
Transport fuel
Phase change/absorption
bulk thermal storage
Heat transfer
Ammonia
Green ammonia production and use.
Direct
combustion
engine/turbine
Directly in
solid oxide
fuel cell
(after cracking)
in PEM fuel cell
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 5

INTRODUCTION
Introduction
Ammonia has had a profound global impact
since the discovery of its synthesis from
hydrogen and nitrogen by Haber and Bosch in
Germany at the beginning of the 20th century.
The key role of ammonia today is as the basic
feedstock for inorganic fertilisers that currently
support food production for around half of the
world’s population
1
.
Ammonia is an efficient refrigerant that has
been used extensively since the 1930s in
industrial cold stores, food processing industry
applications and increasingly in large-scale
air-conditioning. Ammonia is also the key
component in the production of AdBlue for
vehicle NO
x
control, and in the pharmaceutical,
textile and explosives industries.
Current global ammonia production is about 176
million tonnes per year and is predominantly
achieved through the steam reforming of
methane to produce hydrogen to feed into
ammonia synthesis via the Haber Bosch process
(see Chapter 1). Ammonia production is a highly
energy intensive process consuming around
1.8% of global energy output each year (steam
methane reforming accounts for over 80% of the
energy required) and producing as a result about
500 million tonnes of carbon dioxide (about 1.8%
of global carbon dioxide emissions)
2,3,4
. Ammonia
synthesis is significantly the largest carbon
dioxide emitting chemical industry process
(Figure 2). Along with cement, steel and ethylene
production, it is one of the ‘big four’ industrial
processes where a decarbonisation plan must
be developed and implemented to meet the net-
zero carbon emissions target by 2050
5
.
1. Smil V. 2000 Enriching the Earth. ISBN 9780262194495.
2. Institute for Industrial Productivity. Industrial Eciency Technology Database – Ammonia.
3. International Fertiliser Industry Association. 2009 Fertilisers, Climate Change and Enhancing Agricultural
Productivity Sustainably. See https://www.fertilizer.org/Public/Stewardship/Publication_Detail.
aspx?SEQN=4910&PUBKEY=0E80C30A-A407-49D2-86B5-0BAC566D3B26 (accessed 29 May 2019).
4. IEA, ICCA, DECHEMA. 2013 Technology Roadmap – Energy and GHG Reductions in the Chemical Industry
via Catalytic Processes.
5. McKinsey & Company. 2018 Decarbonization of Industrial Sectors: the next Frontier. See https://www.mckinsey.com/~/
media/mckinsey/business%20functions/sustainability/our%20insights/how%20industry%20can%20move%20toward%20
a%20low%20carbon%20future/decarbonization-of-industrial-sectors-the-next-frontier.ashx (accessed 29 May 2019).
Greenhouse gas emissions for selected high production volume chemicals for 2010
4
.
FIGURE 2
BTX – Benzene, Toluene, Xylene (aromatic chemicals). These 2010 numbers are the most recent published figures.
Note: Ammonia production in 2018 was 176Mt and generated around 500 million tonnes of carbon dioxide (per annum).
Production volume (Mt)
Annual GHG emissions (Mt CO
2
-eq)
Acrylonitrile
Styrene
Methanol
Propylene
Ethylene
Ammonia
BTX
0
0
50
100
150
200
250
300
350
140100804020 60 120
160
Current ammonia
production
generates 500
million tonnes of
carbon dioxide.
6 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

INTRODUCTION
In addition to its established uses, ammonia
can be applied as a flexible long-term energy
carrier and zero-carbon fuel. In common with
fossil fuels, ammonia is both a chemical energy
store and a fuel, where energy is released by
the breaking and making of chemical bonds.
For ammonia (NH
3
), the net energy gain arises
from breaking nitrogen-hydrogen bonds which,
together with oxygen, produces nitrogen and
water. Importantly, this means that if sustainable
energy is used to power the production of green
ammonia, it can be made sustainably using only
air (which is around 78% nitrogen) and water.
The energy storage properties of ammonia
are fundamentally similar to those of methane.
Methane has four carbon-hydrogen bonds
thatcan be broken to release energy and
ammonia has three nitrogen-hydrogen bonds
that can be broken to release energy (Figure 3).
The crucial difference is the central atom,
where, when burnt, the carbon atom in
methane produces carbon dioxide, whereas
the nitrogen atom in ammonia results in
nitrogen gas, N
2
.
At room temperature and atmospheric pressure,
ammonia is a colourless, pungent gas. To
store in bulk, it requires liquefaction either by
compression to 10 times atmospheric pressure or
chilling to -33°C. In this state, the energy density
of ammonia is about 3 kWh/litre which is less
than but comparable with fossil fuels (Figure 4).
Hydrogen by comparison is also a gas at
atmospheric pressure and room temperature.
However to store hydrogen at scale it must
be compressed to around 350 to 700 times
atmospheric pressure, or cryogenically cooled
to -253°C. Consequently, the storage of
hydrogen is more difficult, energy intensive
and expensive than storing ammonia.
The volumetric energy density of a range of fuel options.
FIGURE 4
KEY
Carbon-based fuels
Zero-carbon fuels
Diesel
Petrol (octane)
Liquefied Petroleum Gas
Ethanol
Liquefied Natural Gas
Methanol
Ammonia (liquid, -35°C)
Ammonia (liquid, 25°C)
Hydrogen (liquid)
Hydrogen (700bar)
Hydrogen (350bar)
Li-battery (NMC)
0 1 2 3 4 5
Energy density (kWh/l)
6 7 8 9 10
Structure of methane and ammonia.
Methane CH
4
H HC
H
H
H HN
Ammonia NH
3
H
FIGURE 3
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 7

INTRODUCTION
Ammonia shipping infrastructure, including a heat map of liquid ammonia carriers and existing
ammonia port facilities (2017).
FIGURE 5
Current ammonia storage and transport
infrastructure
There is a high level of maturity in many
aspects of ammonia storage and transport
infrastructure because of its widespread
use as a feedstock for inorganic fertilisers.
Indeed, an established worldwide ammonia
infrastructure already exists with significant
ammonia maritime trading. International
shipping routes are well-established and
there is a comprehensive network of ports
worldwide that handle ammonia at large
scale (Figure 5). This existing port and
shipping infrastructure could enable the
early accelerated adoption of large-scale
transportation of ammonia as an energy
vectorand fuel.
The largest refrigerated ammonia storage
facilities are often located at ports where
ammonia is produced and then shipped
internationally. As an indication of scale, the
Qatar Fertiliser Company ammonia production
facility has two 50,000 tonne refrigerated
ammonia storage tanks which have a
combined footprint of around 160m by 90m
(around 1.5 hectares)
6
.
6. McDermott. QAFCO Ammonia Storage Tanks – Snamprogetti. See www.mcdermott.com/What-We-Do/Project-Profiles/
QAFCO-Ammonia-Storage-Tanks (accessed 10 June 2019).
KEY
Ammonia loading facilities Ammonia unloading port facilities
8 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

INTRODUCTION
In the UK, ammonia is used to make nitrate
fertilisers which are applied to the soil, while
in the United States of America, ammonia
is mainly applied directly into the soil.
Consequently, the USA has over 10,000
ammonia storage sites, predominately located
in the Mid-West (Figure 6); though there is a
significant presence of ammonia facilities in
cities such as Los Angeles (storage capacity
of 150,000 tonnes in Port of Los Angeles). The
highest densities are in Iowa with over 1,000
facilities and a total storage capacity of around
800,000 tonnes. Transportation is not only by
road, train and river but also by pipeline; 3,000
miles of 6 – 8-inch carbon steel pipes connect
11 states with regularly spaced pumping
stations, transporting around 2 million tonnes
of ammonia per year
8
.
Liquefied ammonia storage and pipeline distribution networks in the US Mid-West
7
. The Kaneb
(orange line) and Magellan Midstream (red line) ammonia pipelines are respectively 2,000 miles
and 1,100 miles long.
FIGURE 6
7. U.S. Environmental Protection Agency. Facility Registry Service https://www.epa.gov/frs (accessed 05 June 2019).
8. Papavinasam S. 2014 Corrosion control in the Oil and Gas Industry. Gulf Professional Publishing, 2, 41 – 131.
(doi: 10.1016/B978-0-12-397022-0.00002-9).
Note: The Magellan Midstream pipeline will be decommissioned in 2020.
Circle areas are indicative of ammonia tonnage. The largest circles correspond to 100,000 tonne facilities.
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 9

INTRODUCTION
Ammonia: health and environmental
considerations
In considering expanded roles for ammonia in
energy storage, the health risks from ammonia
exposure and the environmental risks arising
from leaks must be closely scrutinised and all
systems must be designed to minimise, and
effectively eliminate, these risks. Ammonia is
corrosive and potentially toxic. Its high vapour
pressure under standard conditions enhances
the risks associated with these hazards.
However, ammonia is readily detectable
by smell at concentrations substantially
below levels that cause any lasting health
consequences.
From an environmental perspective, ammonia
represents a chronic hazard to terrestrial
ecosystems as well as providing an increasing
burden to air pollution. Human activity
has greatly modified the very important
biogeochemical global cycle. The global
industrial synthesis of ammonia along with
combustion sources of nitrogen compounds
are similar in magnitude to the natural global
fixation of atmospheric nitrogen by microbes
insoils and in the oceans (Figure 7).
Agricultural fertilisers account for 80% of
annual ammonia production but only 17% of
that nitrogen is consumed by humans in crops,
dairy and meat products
9
. The remainder
leaches into the soil, air and water causing
widespread biodiversity losses, eutrophication,
and air quality issues from particulate
matter, emissions of greenhouse gases and
stratospheric ozone loss
10
.
Once ammonia has been applied to soils
either from fertilisers or deposited from the
atmosphere, it is transformed, by microbes and
depending on soil conditions, to a range of
other compounds including nitric oxide, nitrous
oxide, and molecular nitrogen.
Although ammonia is itself not a greenhouse
gas, following deposition to soil it may be
converted to nitrous oxide, an important
contributor to radiative forcing of climate. It also
has a substantial indirect impact on climate
through its role in particulate matter. One of
the most significant measures to improve the
resulting air pollution in the UK, and more widely
in Europe, is to minimise agricultural ammonia
emissions, through decreasing deposition
11
. It
is therefore important and essential that any
new applications of ammonia include effective
measures to prevent any additional emissions.
In contrast to fertilisers, nitrogen release from
energy storage applications of ammonia should
be as nitrogen gas only. Stringent controls,
which are already present at all ammonia
storage and relevant industrial sites, must be
in place for ensuring that the risks of ammonia
release and NO
x
formation are negligible.
9. Leach AM et al. 2012 A nitrogen footprint model to help consumers understand their role in nitrogen losses to the
environment. Environ. Dev. 1, 40-66. (doi: 10.1016/j.envdev.2011.12.005).
10. Erisman JW et al. 2013 Consequences of human modification of the global nitrogen cycle. Philosophical Transactions
of The Royal Society B, 368, 20130116. (doi: 10.1098/rstb2013.0116).
11. Vieno M et al. 2016 The sensitivities of emissions reductions for the mitigation of UK PM2.5. Atmos. Chem.Phys.,16,
265-276. (doi:10.5194/acp-16-265-2016).
It is essential that
new applications
of ammonia prevent
any additional
emissions.
10 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

INTRODUCTION
The global fixation of atmospheric nitrogen to reactive forms (ammonia, nitric oxide and nitrogen
dioxide). The orange arrows represent natural processes, mainly Biological Nitrogen Fixation
(BNF), the purple arrows represent anthropogenic sources
12
.
FIGURE 7
Biological
Nitrogen
Fixation
Lightning
Combustion
Fertiliser
production
Agricultural
Biological
Nitrogen
Fixation
Biological
Nitrogen
Fixation
58 Mt ± 50%
120 Mt ± 10%
60 Mt ± 30%
140 Mt ± 20%
5 Mt ± 50%
30 Mt ± 10%
OceanLand
Total nitrogen fixation = 413 Mt NH
3
/year
Total anthropogenic nitrogen fixation = 210 Mt NH
3
/year
12. Fowler D et al. 2013 The global nitrogen cycle in the twenty-first century. Philosophical Transactions of The Royal
Society B, 368, 20130164. (doi: 10.1098/rstb.2013.0164).
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 11

CHAPTER ONE
The decarbonisation
of ammonia production
In this briefing the various methods of
producing ammonia are differentiated using
the following terms:
Brown ammonia
Higher carbon ammonia made using a fossil
fuel as the feedstock
Blue ammonia
Low-carbon ammonia: brown ammonia but
with carbon capture and storage technology
applied to the manufacturing processes.
Green ammonia
Zero-carbon ammonia, made using sustainable
electricity, water and air.
The ammonia produced is the same, it is the
carbon emissions from the processes that
aredifferent
1.1 Current ammonia production process –
brown ammonia
Current commercial ammonia production is
predominately based around the Haber-Bosch
process (Figure 8). This reaction involves the
catalytic reaction of hydrogen and nitrogen at
high temperature and pressure.
Overall, brown ammonia production is energy
intensive, consuming 8 MWh of energy per
tonne of ammonia. However, most of the energy
consumption and around 90% of the carbon
emissions are from the production of hydrogen.
Hydrogen is generated almost exclusively via
steam reformation of fossil fuels. Most ammonia
plants rely on the steam reformation of natural
gas to produce hydrogen and carbon dioxide
13
(Figure 9). Coal, heavy fuel oil and naphtha can
also be used but have higher carbon dioxide
emissions (between 2.5 and 3.8 tonnes CO
2
/
tonne ammonia compared to 1.6 tonnes CO
2
/
tonne ammonia for natural gas
14
). The nitrogen
is obtained from compressed air or an air
separation unit.
13. International Fertiliser Industry Association. 2009 Fertilisers, Climate Change and Enhancing Agricultural
Productivity Sustainably. See https://www.fertilizer.org/Public/Stewardship/Publication_Detail.
aspx?SEQN=4910&PUBKEY=0E80C30A-A407-49D2-86B5-0BAC566D3B26 (accessed 29 May 2019).
14. Brightling J. 2018 Ammonia and the Fertiliser Industry: The Development of Ammonia at Billingham. Johnson Matthey
Technol. Rev., 62, 32. (doi: 10.1595/205651318x696341).
Schematic of the Haber Bosch ammonia synthesis reaction.
FIGURE 8
Ammonia
Nitrogen
Hydrogen
CATALYST
150 – 300 bar
350 – 500 °C
Hydrogen
accounts for
around 90%
of the carbon
emissions in
the synthesis
ofammonia.
12 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

CHAPTER ONE
Reducing the amount of carbon dioxide
produced during the manufacturing process
is dependent primarily on the source of
hydrogen, using low-carbon energy for the
process and system integration to produce the
most efficient overall process.
The recent Royal Society Policy Briefing
Options for producing low-carbon hydrogen at
scale
15
and the Committee for Climate Change
report Hydrogen in a low-carbon economy
16
discuss future scenarios for low-carbon
hydrogen production.
Both reports note that the most likely
optionsare:
• Blue hydrogen
Steam methane reforming with carbon
capture and storage (CCS).
• Green hydrogen
Electrolysis of water, to generate hydrogen
and oxygen in a process driven by
sustainable energy.
15. The Royal Society. 2018 Options for producing low-carbon hydrogen at scale: Policy Briefing. See https://royalsociety.
org/-/media/policy/projects/hydrogen-production/energy-briefing-green-hydrogen.pdf (accessed 17 April 2019).
16. Committee on Climate Change, 2018 Hydrogen in a low-carbon economy. See https://www.theccc.org.uk/wp-content/
uploads/2018/11/Hydrogen-in-a-low-carbon-economy.pdf (accessed 29 May 2019).
Schematic of hydrogen production via steam methane reformation.
Desulfurisation
FIGURE 9
Hydrogen
Carbon
dioxide
Hydrogen
Natural
gas
Carbon
dioxide
removal
Primary
reformation
Secondary
reformation
Shift
reaction
Final
purification
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 13

CHAPTER ONE
1.2 Blue ammonia production – using blue
hydrogen from steam methane reforming
(SMR) with carbon capture and storage (CCS)
While natural gas prices and carbon taxes
remain low, steam methane reforming with
carbon capture and storage is likely to be
the lowest cost option for reducing the
carbon footprint of ammonia production
(CCSis estimated to add £0.40/kgH
2
)
17
. Steam
methane reforming emits carbon dioxide in
a concentrated form that is well-suited for
carbon capture and storage. However, the
incorporation of carbon capture technologies
into the steam methane reforming process
has been modelled and shows an increase in
natural gas consumption and a consequent
increase in the operating cost of hydrogen
production relative to the existing process
18
.
While up to 90% of carbon dioxide could
be captured, the upstream greenhouse gas
emissions associated with natural gas extraction,
limit the life-cycle emission reductions for
combined steam methane reforming and
carbon capture and storage to 60 – 85%
19
.
This degree of carbon emission reduction is
impressive but, for net-zero carbon hydrogen
production, current projections suggest that
this process can only be part of a transition to
a zero-carbon solution. This becomes highly
relevant if there is a substantial increase
in hydrogen and ammonia production
associatedwith sustainable energy storage.
1.3 Green ammonia production – using green
hydrogen from water electrolysis
In this process, hydrogen is produced through
the electrolysis of water, which is a well-
established process (Figure 10)
20
. Nitrogen
is obtained directly from air using an air
separation unit which accounts for 2 – 3%
of the process energy used. Ammonia is
produced using the Haber-Bosch process
powered by sustainable electricity.
The main challenges are cost, of which about
85% is electricity, which in most parts of the
world is still significantly more expensive than
natural gas. The International Energy Agency
estimate that electrolysis is cost competitive
with steam methane reforming with carbon
capture at electricity prices between 1.5 to
5.0 USD cents/kWh (1.2 to 4.0 GBP pence/
kWh); and with steam methane without carbon
capture at 1 to 4 USD cents/kWh (0.8 to 3.1 GBP
pence/kWh; assuming gas prices 3 to 10 USD
cents/MMBtu (2.3 to 7.7 GBP pence/MMBtu))
21
.
17. International Energy Agency. 2019 The Future of Hydrogen. See https://www.iea.org/hydrogen2019/
(accessed 29 May 2019).
18. International Energy Agency Greenhouse Gas R&D Programme. 2017 Techno-Economic Evaluation of SMR Based
Standalone (Merchant) Hydrogen Plant with CCS. See https://ieaghg.org/exco_docs/2017-02.pdf
(accessed 23 May 2019).
19. Committee on Climate Change, 2018 Hydrogen in a low-carbon economy. See https://www.theccc.org.uk/wp-content/
uploads/2018/11/Hydrogen-in-a-low-carbon-economy.pdf (accessed 29 May 2019).
20. The Royal Society. 2018 Options for producing low-carbon hydrogen at scale: Policy Briefing.
See https://royalsociety.org/-/media/policy/projects/hydrogen-production/energy-briefing-green-hydrogen.pdf
(accessed 17 April 2019).
21. International Energy Agency. 2019 The Future of Hydrogen. See https://www.iea.org/hydrogen2019/
(accessed 29 May 2019).
14 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE
CHAPTER ONE
Schematic of green ammonia production based upon hydrogen production from water
electrolysis and the full decarbonisation of the Haber-Bosch process.
FIGURE 10
Water
Air
Electrolysis
Separation
Sustainable
electricity
HABER-
BOSCH
PROCESS
Ammonia
Sustainable
electricity
Hydrogen
Nitrogen
22. Kruger K, Eberhard A, Swartz K. 2018. Renewable Energy Auctions: A Global Overview. See http://www.gsb.uct.ac.za/
files/EEG_GlobalAuctionsReport.pdf (accessed 17 April 2019).
23. International Renewable Energy Agency. 2017 Levelised costs of electricity (LCOE) 2010-2017. See www.irena.org/
Statistics/View-Data-by-Topic/Costs/LCOE-2010-2017 (accessed 23 May 2019).
24. Ash N, Scarbrough T. 2019 Sailing on Solar: Could green ammonia decarbonise international shipping? Environmental
Defense Fund. See https://europe.edf.org/file/399/download?token=agUEbKeQ (accessed 23 May 2019).
The cost of electricity in areas with abundant
renewable potential has decreased
dramatically over the past decade. Auction
prices of 4.5, 3.2 and 2.3 USD cents/kWh
(3.4, 2.5 and 1.7 GBP pence/kWh) for utility-
scale solar installations in Morocco, Chile and
Saudi Arabia respectively, indicate that water
electrolysis may already be cost competitive
with steam methane reforming with carbon
capture and storage in areas with optimal
renewable energy conditions
22,23
. Taking
advantage of such low electricity costs also
requires transporting hydrogen affordably on
a massive scale. Ammonia, with its existing
high degree of technological readiness, is
positioned to play a key role in this supply
chain
24
. The production of green ammonia
viaelectrolysis is operating at TRLs 5 – 9.
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 15

CHAPTER ONE
Figure 11 shows that the lowest current costs
of green ammonia production are already
competitive with blue ammonia. However, the
Figure also reflects how the present costs
of ammonia production vary widely across
different regions due to variations in fuel and
feedstock costs. This is especially evident
for production via electrolysis where the
cost of electricity is a major factor; the lowest
electrolysis costs are from locations where
renewable electricity costs are the lowest,
which, globally, is solar, from areas of high
global horizontal irradiance and onshore wind.
In 2019, the UK strike price for future offshore
wind dropped toaround 4.0 GBP pence/kWh
(5.2 USD cents/kWh).
1.3.1 Research opportunities
Demonstration projects for large-scale
green ammonia production are likely to be
electrolysis-based and sited in areas with
abundant renewable electricity, such as
North-Western Australia (see Chapter 3).
The development of new small-scale plant
designs which couple electrolysis with
ammonia production (see Case study 1) are
also under development. The opportunity to
combine smaller scale ammonia production
with remote renewable generation is attractive,
if lower capital costs can be realised. To
enable ammonia to be produced at this scale,
adaptation will be required to operate at a
sub-megawatt scale. The downscaling of the
Haber-Bosch process to small (30 – 500kW)
intermittent and variable renewable energy
supplies introduces two principal challenges:
• potential degradation of catalyst
performance and reduction of catalyst
lifetime from changes to Haber Bosch
reactor temperature and pressure because
of intermittent operation,
• minimisation of the inevitable efficiency loss
in moving to smaller scale and non-steady
state operation.
There are several recent demonstrations
of ammonia production by a downscaled
Haber-Bosch process integrated with water
electrolysis: one such trial is in operation at the
Rutherford Appleton Laboratory in Oxfordshire
(see Case study 1). A similar 20kg ammonia per
day demonstrator in Fukushima, Japan, which
is also powered by wind, has been designed to
test the cycle stability of different catalysts
25
.
Energy supply intermittency may be
circumvented by consuming small amounts
of ammonia product to maintain constant
temperature and pressure when required.
Such small-scale distributed ammonia
production could be used locally (eg
generated on a farm and used on the land)
or collected and transported by tanker to a
regional energy store.
25. Cross-ministerial Strategic Innovation Promotion Program. 2018 Press Release – World’s first successful ammonia
synthesis using renewable energy-based hydrogen and power generation. See www.jgc.com/en/news/assets/
pdf/20181019e.pdf (accessed 10 June 2019).
16 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

CHAPTER ONE
Cost comparison of ammonia production via different methods
26
.
FIGURE 11
Note: Range refers to the range of total levelised costs across regions, the lower end of the range is disaggregated into cost
categories. Electrolysis is assumed to be powered by 100% renewable electricity; the ‘feedstock cost’ is the electricity for the
electrolyser, and ‘fuel cost’ is additional electricity for the air separation unit, synthesis loop etc. CCUS costs include capture,
transport and storage of carbon dioxide; process CCUS is only process emissions; total is process and energy related
emissions. % carbon dioxide reduction is relative to unabated production with natural gas (1.6 tonnes/tonne NH
3
).
1,200
Natural Gas
unabated
Natural Gas
with concentrated
process CCUS
Natural Gas
with concentrated
total CCUS
Electrolysis
no direct emissions
Brown Ammonia Blue Ammonia Blue Ammonia Green Ammonia
Levelised costs (£/t ammonia)
CO
2
emissions (tonnes/tonne NH
3
)
0 0
0.2
0.4
0.6
0.8
1
1.2
1.4
1.6
1.8
200
400
600
800
1,000
1,400
1,600
1,800
2,000
KEY
CCUS costs Feedstock Fuel OPEX CAPEX CO
2
emissions Cost range
26. International Energy Agency. The Future of Hydrogen, June 2019 https://webstore.iea.org/the-future-of-hydrogen
(accessed Oct 2019)
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 17

CHAPTER ONE
Downscaling Haber-Bosch
A team comprised of Siemens plc, Cardiff University, the University of Oxford and the
Science & Technology Facilities Council (STFC), have developed a green ammonia
energy demonstration system at the Rutherford Appleton Laboratory, Oxfordshire
27
.
Thisdemonstrator is designed to show feasibility and round trip efficiency through in-situ
synthesis, storage and combustion of green ammonia. An on-site wind turbine generates
the electricity to power both water electrolysis and the Haber-Bosch process. This system
produces around 30kg/day of ammonia which is stored in a pressurised tank and a 30kW
sparkignition electric generator uses this ammonia to feed electricity back into the grid.
CASE STUDY 1
Image
Green ammonia
demonstration system,
Rutherford Appleton
Laboratory, Oxfordshire.
27. The Chemical Engineer. Green ammonia project set for launch in UK today. See https://www.thechemicalengineer.
com/news/green-ammonia-project-set-for-launch-in-uk-today/ (accessed 29 May 2019).
18 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

CHAPTER ONE
While these demonstration projects indicate
the relative technological readiness of green
ammonia production, there are substantial
opportunities for improvements in efficiency
andcost reductions. As such, research efforts
will play an important role in shaping the future
of green ammonia production technologies.
Some key goals include:
• Developing more active Haber Bosch
catalysts will facilitate operation under
milderconditions and reduce energy
demand, making the process more
amenableto variable and smaller-scale
operation and improving its compatibility
with renewable electricity. Reductions in
the operating pressure to levels where
expensivecompressors are not required
would be a valuable advance. This research
and development ranges from TRLs 1 – 4.
• Ammonia separation methods: sequestering
ammonia as it is produced by the use of
ammonia absorption materials may facilitate
lower-pressure operation and higher
ammonia yields in Haber-Bosch units.
Research, development and demonstration
are at TRLs 1 – 5.
1.4 Novel methods for green ammonia
synthesis
In addition to the Haber-Bosch process,
there are other recognised methods of green
ammonia production. All are still operating at
abasic research stage:
1. Ammonia is produced naturally by bacteria
that contain an enzyme catalyst called
nitrogenase, which operates at room
temperature and pressure to synthesise
ammonia from water and nitrogen.
Althoughbiological nitrogen fixation
is a perfect source of green ammonia,
further research and development would
be required before large-scale industrial
production could be considered. This
processis currently at TRL 1.
2. Electrochemical production is a technology for
producing green ammonia directly from water
and nitrogen using electricity. Importantly there
is no separate hydrogen production process
step. This process would be ideal for distributed
(small-scale) generation and more amenable
to intermittent power supplies (see Case
study 2). However, to date, only low rates of
ammonia production have been demonstrated
in laboratory studies. New electrocatalysts,
electrolytes and systems must be developed
that can produce ammonia in preference to
hydrogen and achieve competitive production
rates
28,29
. This process is currently at TRLs 1 – 2.
3. Chemical looping processes involve a series
of chemical/electrochemical reactions which
produce ammonia as a by-product, but where
the core reaction chemicals are recycled
and are not lost
30,31,32
. These processes
may be attractive for intermittent operation.
Importantly, some of these cycles avoid the
need for a separate hydrogen production
process by reacting with water directly. This
process is operating at TRLs 1 – 4.
28. Giddey S, Badwal SPS, Kulkarni A. 2013 Review of electrochemical ammonia production technologies and materials.
International Journal of Hydrogen Energy, 38, 14576–14594. (doi: 10.1016/j.ijhydene.2013.09.054).
29. Kyriakou V et al. 2017 Progress in the Electrochemical Synthesis of Ammonia. Catalysis Today, 286, 2–13. (doi:
10.1016/j.cattod.2016.06.1014).
30. McEnaney JM et al. 2017 Ammonia synthesis for N2 and H2O using a lithium cycling electrification strategy at
atmospheric pressure. Energy & Environmental Science,10, 1621–1630 (doi: 10.1039/C7EE01126A).
31. Gao W et al. 2018 Production of Ammonia via a Chemical Looping Process Based on Metal Imides as Nitrogen
Carriers. Nature Energy, 3, 1067–1075 (doi: 10.1038/s41560-018-0268-z).
32. Hargreaves JSJ. 2014 Nitrides as ammonia synthesis catalysts and as potential nitrogen transfer reagents. Applied
Petrochemical Research, 4, 3–10 (doi: 10.1007/s13203-014-0049-y).
There are other
recognised
methods of
green ammonia
production.
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 19

CHAPTER ONE
Process development – Solid Oxide Electrolysis Cell (SOEC)
Haldor Topsoe are developing a demonstrator that integrates a solid oxide electrolysis cell
(SOEC) to produce ammonia synthesis gas (H
2
:N
2
= 3:1). This is then converted to ammonia
via the conventional Haber-Bosch process (Figure 12). The process operates at high
temperatures and can separate oxygen from air without using an air separation unit (ASU).
This results in an expected energy consumption per tonne of ammonia that is 5 – 10% lower
than a conventional SMR-based process and even less than a SMR-based process with
carbon capture and storage.
The waste heat is used to increase the overall efficiency to over 70% of the lower heating
value (LHV) of ammonia. The high overall efficiency and lower investment costs (ASUs are
expensive at small scale) improve the economics of small-scale ammonia production.
Haldor Topsoe recently announced the commencement of the SOC4NH3 project (Solid
Oxide Cell based production and use of ammonia), which will feature a 50kW demonstration
plant using this new technology combination, with the aim of commercial availability in 2030.
CASE STUDY 2
Process flow diagram of ammonia synthesis using a Solid Oxide Electrolysis Cell (SOEC)
toproduce both hydrogen and nitrogen for the Haber Bosch process.
FIGURE 12
Water
Water
Air
Oxygen
HABER-
BOSCH
SYNTHESIS
Ammonia
SOEC
Steam
Hydrogen
Nitrogen
Sustainable
electricity
20 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

CHAPTER TWO
EXPANDED USES
EXISTING USES
New zero-carbon uses for
green ammonia
In addition to decarbonising the existing
uses of ammonia, the development of green
ammonia production also generates the
following additional uses (Figure 13):
• Ammonia can be used as a medium to
store and transport chemical energy, with
the energy being released either directly
(see Chapter 2.1) or by the full or partial
decomposition of ammonia to release
hydrogen (see Chapter 2.2). The hydrogen or
ammonia-hydrogen mixture is then reacted
with oxygen in the air to release energy.
• Ammonia can be used as a transport fuel
by direct combustion in an engine or by
chemical reaction with oxygen in a fuel cell
to produce electricity to power a motor
(seeChapter 2.3).
• Ammonia can also be used to store
thermalenergy through for example liquid
to gas phase changes, solid to solid phase
transformations and absorption with, for
example, water (see Chapter 2.4).
Schematic of existing and expanded end uses of ammonia.
FIGURE 13
PEM – Proton Exchange Membrane.
Fertilisers
Refrigeration
Explosives
Textiles and
pharmaceuticals
Ammonia
Direct
combustion
engine/turbine
Directly in
solid oxide
fuel cell
(after cracking)
in PEM fuel cell
Transport fuel
Phase change/absorption
bulk thermal storage
Heat transfer
Ammonia can
be used as a
medium to store
and transport
chemical energy.
Energy store to electricity generation
(after cracking)
in PEM fuel cell
Using
alkaline
fuel cell
Direct
combustion
engine/turbine
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 21

CHAPTER TWO
2.1 The storage and transportation of
sustainable energy
The energy flow in a zero-carbon economy
begins with the generation of primary
electricity from sustainable energy sources.
Once generated, this energy must either be
used immediately or stored. There are several
ways of storing and recovering zero-carbon
energy that include:
• electrochemical storage in batteries,
• physical storage in, for example, pumped
hydroelectricity and compressed gases,
• chemical storage in the form of zero-carbon
electrofuels, such as hydrogen or ammonia
Each zero-carbon storage option has its
relative merits in terms of flexibility, efficiency,
energy density, cost, scale and longevity.
While it is always preferable to keep energy
transitions to a minimum (see Chapter 2.5),
additional considerations such as the energy
and financial costs of storage and transport
must also be considered. For example,
it mightbe preferable to store and use
hydrogenlocally rather than convert it to
ammonia if local, low-cost, large-scale gas
storage (eg in salt caverns) is available.
Ammonia, with its relatively high energy
density and existing global transportation
and storage infrastructure, could offer a new,
integrated worldwide sustainable energy
storage and distribution solution (see, for
example, Case study 3)
33
. The relative ease
of storing liquid ammonia either compressed
or refrigerated, particularly compared with
compressed or liquefied hydrogen, makes
ammonia a competitive option for storing zero-
carbon energy and transporting it by pipeline,
road, rail or ship (Figure 14 and Figure 15).
33. Thyssenkrupp – WattshiftR. 2018 WattshiftR concept – oshore energy & ammonia production.
22 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

CHAPTER TWO
Estimated costs for transport of hydrogen and ammonia by lorry, rail and ship
34
.
FIGURE 14
KEY
Ammonia Liquid hydrogen 350 bar hydrogen
Shipping
Rail
Lorry
0 0.2 0.4 0.6 0.8 1
Cost (£/tonne km)
1.2 1.4 1.6 1.8
34. ACIL Allen Consulting. 2018 Opportunities for Australia from Hydrogen Exports. See https://www.acilallen.com.au/
projects/energy/opportunities-for-australia-from-hydrogen-exports (accessed 23 May 2019).
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 23
CHAPTER TWO
0
200 800 1,400
km
Cost (£/kgH
2
)
2,000 2,600
0.2
0.4
0.6
0.8
1
1.2
1.4
1.6
1.8
Cost estimates for transport of energy as hydrogen or ammonia by ship and pipeline
35
.
FIGURE 15
Hydrogen (pipeline)
Hydrogen (ship)
Ammonia (pipeline)
Ammonia (ship)
Several recent studies have concluded that
ammonia is the lowest-cost method and
the most technologically-ready option for
transporting energy over long distances
(Figure 15). The cost of converting hydrogen
to ammonia is around £0.80/kgH
2
, so for
example, the total cost of transporting ammonia
1,400km by pipeline is £1.20/kgH
2
. The cost of
transporting hydrogen by pipeline increases
faster than ammonia, so from around 2,500km
the costs are both around £1.50/kgH
2
(including
the conversion cost); beyond this distance,
ammonia is cheaper.
The direct use of ammonia, for example,
in a direct ammonia solid oxide fuel cell or
internal combustion engine, brings significant
increases in both energy efficiency and
reduced energy costs. There are 4,830km
ofammonia pipelines in the United States and
in Eastern Europe, the Tolyatti-Odessa pipeline
(2400km) transports ammonia from Russia to
chemical and fertiliser plants
36
.
Storage cost estimates are expected to be
comparable with, for example, the storage of
hydrogen in salt caverns, but with the added
advantages of flexibility of scale, location and
onward transportation. The cost of refrigerated
ammonia storage tanks varies depending
upon the size, site and the facilities available,
with estimates for a 10,000 tonne standalone
storage tank costing between £20 – 40million.
The UK has a developed understanding of the
safe handling and storage of ammonia which
should permit the appropriate infrastructure to
be developed.
35. International Energy Agency. The Future of Hydrogen, June 2019 https://webstore.iea.org/the-future-of-hydrogen
(accessed 24 October 2019).
36. International Energy Agency. The Future of Hydrogen, June 2019 https://webstore.iea.org/the-future-of-hydrogen
(accessed 24 October 2019).
Note: Hydrogen transported via pipeline is gaseous and liquefied for shipping. Costs include both the transport and storage
required; not the conversion, distribution or reconversion.
24 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

CHAPTER TWO
37. Wind Europe. 2019 Oshore Wind in Europe. See windeurope.org/wp-content/uploads/files/about-wind/statistics/
WindEurope-Annual-Oshore-Statistics-2018.pdf (accessed 23 May 2019).
38. TenneT. North Sea Wind Power Hub. See www.tennet.eu/our-key-tasks/innovations/north-sea-wind-power-hub/
(accessed 23 May 2019).
39. Jepma CJ, van Schot M. 2017 On the economics of oshore energy conversion: smart combinations. Energy Delta Institute.
40. Meier K. 2014 Hydrogen production with sea water electrolysis using Norwegian oshore wind energy potentials.
International Journal of Energy and Environmental Engineering, 5, 104 (doi: 10.1007/s40095-014-0104-6).
41. Thyssenkrupp – WattshiftR. 2018 WattshiftR concept – oshore energy & ammonia production.
42. Oil & Gas Authority. 2019 Cost Estimate Report: UKCS Decommissioning. See https://www.ogauthority.co.uk/
media/5906/decommissioning-estimate-cost-report-2019.pdf (accessed 26 November 2019).
Storing and transporting renewable energy
The dramatic reductions in electricity costs
from both onshore and offshore wind farms
are advantageous for North Western Europe,
and in particular for the UK. The North Sea
accounts for 70% of all offshore wind capacity
in Europe
37
and by 2040, offshore wind
turbines in the North Sea are expected to
generate 70 – 150GW of electricity; around
20% of the EU’s electricity demand
38
. Utilising
renewable electricity to produce ammonia will
enable more flexible options for renewable
energy in the energy economy.
The Energy Delta Institute and the Energy
Research Centre of the Netherlands
completed a feasibility study of
offshore green hydrogen production on
decommissioned oil/gas platforms in the
North Sea linked to offshore wind farms
39
.
Hydrogen would be transported onshore
via existing gas pipelines (after mixing with
natural gas) or by building new hydrogen
pipelines. They assessed all costs associated
including energy conversion, storage and
transport. Using various assumptions for the
output and input variables and based on
market data at the time, the green hydrogen
prices ranged between €1.56 – 4.67/kgH
2
(£1.33 – 3.98/kgH
2
). Electrolyser capacity was
also assessed for different sized platforms.
Offshore array cables to connect the wind
farms to the platforms cost around €465
(£396) (800m
2
array cable) to €180 (£153)
(240mm
2
array cable) per metre (installation
costs of €200/metre (£170/metre)). Optimal
transport modes of the hydrogen between
the platforms and shore were dependent
on the required distance. This study
involved afresh water infeed for electrolysis,
howeverother studies are considering
seawater electrolysis
40
.
ThyssenKrupp have extended this concept
to explore the production of ammonia as
both an energy store or hydrogen carrier
41
.
To note, there are currently over 600
offshore oil and gas installations in the
North Sea (470 in UK waters). A large
proportion have now exceeded or are
approaching end of designed lifespan
and will be decommissioned in line with
regulation. Recent estimates from the Oil
& Gas Authority show the total cost of
decommissioning remaining UK offshore
oil and gas production, transportation and
infrastructure are £51 billion
42
.
CASE STUDY 3
Image
North Sea oil platform
© jgshields.
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 25

CHAPTER TWO
2.2 Ammonia for the transportation and
provision of hydrogen
The safe, effective, economical and regulated
storage of hydrogen for use as a fuel in
road transport is an important technological
challenge in the move towards a low-carbon
economy. When liquefied, ammonia contains
50% more hydrogen by volume than liquid
hydrogen. These properties, along with ease
of storage and transportation, make ammonia
an attractive candidate for consideration
for the storage and delivery of hydrogen
for hydrogen fuel cell vehicles, with its high
hydrogen content of 17.8wt%.
Ammonia can be straightforwardly decomposed
or ‘cracked’ into nitrogen and hydrogen gases
(Figure 16). The optimal catalytic decomposition
of ammonia is critical. It can be achieved at high
temperatures above 700°C using inexpensive
materials such as iron. Lower temperature
decomposition reduces energy costs but
currently involves the use of rare-metal catalysts
such as ruthenium. Further cost reductions and
optimisation of catalyst and reaction processes
will be required to ensure that energy losses
from the ammonia decomposition reaction are
close to the theoretical minimum value of about
7% of the stored energy of ammonia. Active
research and innovation programmes, including
in the UK, show significant promise with new
inexpensive catalyst families, based on amide
(-NH
2
) and imide (-NH) materials, that operate at
450 – 500°C
43,44
. This is currently at TRLs 2 – 4.
43. David WIF et al. 2014 Hydrogen Production from Ammonia Using Sodium Amide. J Am Chem Soc,136, 13082–13085.
(doi:10.1021/ja5042836).
44. Makepeace JW, Wood TJ, Hunter HMA, Jones MO, David WIF. 2015 Ammonia decomposition catalysis using non-
stoichiometric lithium imide. Chem Sci, 6, 3805–3815. (doi:10.1039/C5SC00205B).
Ammonia
contains 50%
more hydrogen
by volume than
liquid hydrogen.
26 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

CHAPTER TWO
Hydrogen
CATALYST
Its high hydrogen content and established
storage and transportation options make
ammonia an attractive source of hydrogen. The
hydrogen generated from the decomposition
of ammonia can be used to generate electricity,
typically today using proton exchange
membrane (PEM) fuel cells. PEM fuel cells can
be used to power vehicles and also for static
isolated and grid electricity generation.
They are, however, sensitive to very low levels
(<1ppm) of ammonia in the hydrogen gas
stream; for both environmental and process
reasons, the ammonia limits must be kept
below 1ppm. Post-cracking purification is
therefore a critical technical step for obtaining
a usable hydrogen stream. Several approaches
are being developed to purify the hydrogen
stream produced from ammonia decomposition
before its use in a PEM fuel cell, including
membranes
45
and absorption-based systems
46
.
Cracking ammonia to hydrogen to be used in a Proton Exchange Membrane (PEM) Fuel Cell.
FIGURE 16
Ammonia
PURIFICATION
PEM
fuel cell
+trace ammonia
Hydrogen
Nitrogen
45. Lamb KE et al. 2018 High-Purity H2 Produced from NH3 via a Ruthenium-Based Decomposition Catalyst and
Vanadium-Based Membrane. Ind. Eng. Chem. Res, 57, 8–13. (doi: 10.1021/acs.iecr.8b01476).
46. van Hassel BA et al. 2015 Ammonia sorbent development for on-board H2 purification. Separation and Purification
Technology, 142, 215-226. (doi: 10.1016/j.seppur.2014.12.009).
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 27

CHAPTER TWO
2.3 Technological opportunities for ammonia
as a transport fuel
There are several power technologies that
work well with ammonia (or ammonia-derived
hydrogen) as an energy source. Ammonia
can be reacted with oxygen from the air in
a fuel cell to produce electricity or it can be
burned in internal combustion engines and
gas turbines. All uses have their advantages,
challenges and requirements for research
and development (see Table 1).
Technology (efficiency) Required pre-treatment Capital Cost (£/kW)
Proton exchange membrane
(PEM) fuel cell
(40 – 50%)
Ammonia decomposition
Trace ammonia removal
100 (mobile)
1,300 (stationary)
Alkaline fuel cell (AFC)
(50 – 60%)
None 1300 (stationary)*
Solid oxide fuel cell (SOFC)
(50 – 65%)
None 760 (stationary)
Internal combustion engine (ICE)
(30 – 40%)
Ammonia can be used directly but
partial decomposition is beneficial
30 – 45 (mobile)
1,000 (stationary)
Boilers and Furnaces
(85 – 90%)
None 150 – 350 (stationary)
Combined cycle gas turbine (CCGT)
(55 – 60%)
Ammonia can be used directly but
partial decomposition is beneficial
750 (stationary)
Fuel technologies applicable to ammonia.
TABLE 1
Estimated cost values for mobile and static applications are based on current technologies that are under development.
*Currently, there are no AFCs that would be close to technological readiness for a mobile system. For stationary systems,
from a raw materials viewpoint, it would be expected that the price would be competitive with other fuel cell systems,
but only two companies are currently developing them, so data is not available on these systems.
Ammonia can be
burned in internal
combustion
engines.
28 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

CHAPTER TWO
TABLE 1
Advantages Challenges R&D Focus
Established technology
Suitable for mobile applications
Cost and use of platinum
Sensitive to un-cracked ammonia
Trend for decreasing platinum use
Development of ammonia technologies
Non-use of platinum
(or similar metals)
Highly tolerant of ammonia
Low energy density
Few commercial suppliers
Requires carbon dioxide
scrubbing
Increase in energy density
Improve suitability for stationary applications
Innovation for carbon dioxide scrubbing
Direct ammonia systems
Established technology
Decomposes ammonia in-situ
Non-use of platinum
(or similar metals)
High temperatures of operation
Large-scale commercialisation
Corrosion of components
Improve suitability for stationary
applications, combining heat and power
Investigate for transportation applications
Reduction of oxidation impacts
Established technology with other
fuels (ie ammonia mixtures with
gasoline, diesel, hydrogen, etc.)
Robust technology
High power density
Pure ammonia combustion still
under development
NO
x
gases and ammonia slip need
to be limited
Low efficiency
Development of novel ammonia
cracking systems
Ammonia can be used to remove NO
x
gases
Improved combustion technologies to
fully burn ammonia
Established technology at low
ammonia content (up to 20 wt%)
Very robust technology
High power outputs (>1MW)
Increase in ammonia content
Reduction of ammonia slip
NO
x
gases need to be limited
Corrosion caused by aggressive
atmospheres
Improvement in injection and
combustiontechnologies
Development of new systems that
use new materials and innovative
distributionconcepts
High power outputs (>1MW)
Support to produce power
during peak consumption times
Full cycle development
(heat, power, cooling)
Ammonia complete combustion still
under development
NO
x
gases need to be limited
Technology under development:
NO
x
gases are required to
belimited
Development of new combustors for
efficient burning
Ammonia can be used to remove NO
x
gases during combustion
Global (Japan) efforts to design a large
power unit by 2030
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 29

CHAPTER TWO
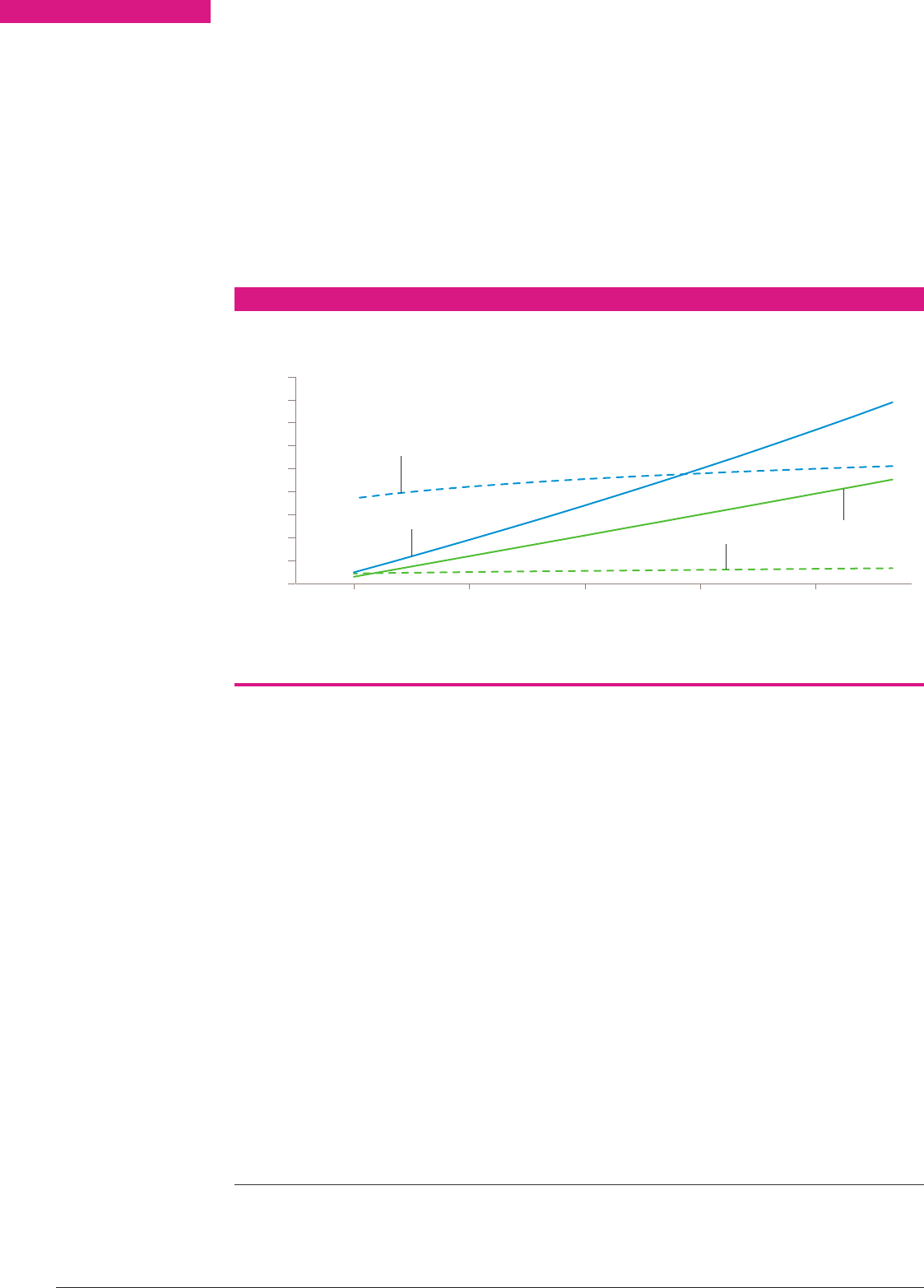
Figure 17 shows a comparison of various
fuels(including storage weights and
efficiencies) for mobile applications using a
range of different energy sources. Although
hydrocarbon fuels store more energy, the
greater efficiency of ammonia powered fuel
cells means that, for example, direct ammonia
fuel cells have a similar overall performance
to liquid propane gas (LPG) powered internal
combustion engines. Potential alternative
low-carbon energy vectors, such as lithium
batteries and liquid-to-gas expansion systems,
have a much lower energy density than all
chemical storage options and their suitability
is dependent on the energy demands of
thejourney.
Specific energy and energy density of a range of energy stores for mobile applications
accounting for typical container properties and energy conversion technology efficiencies.
FIGURE 17
ICE – Internal combustion engine, FC – fuel cell, LPG – liquid propane gas.
Energy per unit mass (kWh/kg)
Energy per unit volume (kWh/l)
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0
0.0
0.5
1.0
1.5
2.0
2.5
Nitrogen (liquid)
Li battery (Nissan Leaf)
Ammonia (ICE)
Methane (250 bar)
Hydrogen (700 bar)
Methanol (FC)
Methane (liquid)
Ethanol
LPG
Ammonia (FC)
Petrol
Diesel
Hydrogen (liquid)
30 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

CHAPTER TWO
Ammonia is a suitable fuel for transport modes
where large amounts of energy are required
for extended periods of time and where
batteries or direct electrical connection are not
practical or cost effective. Examples include
heavy good vehicles, trains, aviation and
shipping (see Case study 4). The MAN Energy
Solutions’ demonstration programme to retrofit
current liquid natural gas marine engines
to run on ammonia, offers an economically
feasible route toward the decarbonisation of
large-scale maritime transportation. Progress
in the modification of internal combustion
engines and gas turbines to run on ammonia
similarly offers a viable transition that is based
around retrofitting current technologies which
impact both transportation and electricity
production. Combustion of ammonia may also
help meet industry requirements for process
heat in areas which are difficult to electrify,
fulfilling a role which is currently played by
fossil fuels. Similarly, ammonia could also be
used to provide green hydrogen for low-
carbon steelmaking methods
47
.
Direct ammonia solid-oxide fuel cells offer a
high efficiency route both for transportation
and future electricity production. Advances
in solid oxide fuel cells, for example from the
NASA Glenn Research Center
48
, have led to
high specific and volumetric power densities of
up to 2.5kW/kg and 7.5kW/l that are sufficient
to power unmanned aerial vehicles and have
the potential to facilitate the reduction of
carbon emissions from aviation.
47. International Energy Agency. 2017 Renewable Energy for Industry. See https://www.iea.org/publications/insights/
insightpublications/Renewable_Energy_for_Industry.pdf (accessed 24 October 2019).
48. NASA Technology Transfer Program. 2017 High Power Density Solid Oxide Fuel Cell. See https://ntts-prod.
s3.amazonaws.com/t2p/prod/t2media/tops/pdf/LEW-TOPS-120.pdf (accessed 10 October 2019).
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 31

CHAPTER TWO
49. Giddey S, Badwal SPS, Munnings C, Dolan M. 2017 Ammonia as a Renewable Energy Transport Media. ACS
Sustainable Chem Eng., 5, 10231-10239. (doi:10.1021/acssuschemeng.7b02219).
2.4 The use of ammonia in heating
andcooling
In addition to using ammonia combustion as
a source of heat, ammonia can also store
and release significant energy on changing
between its liquid and gas forms (1371.2 kJ/kg
at atmospheric pressure). It has the potential to
become significant in the decarbonisation of
space heating and cooling. Star Refrigeration,
based in the UK, has recently developed and
installed heat pumps based on ammonia, that
can use low-grade waste heat to generate
heated water up to 90°C.
Ammonia can also be used in thermochemical
heat storage systems, where the reversible
reaction of ammonia and a metal salt can be
used to store and release heat. These systems
are at a proof-of-concept stage and could find
practical application in long-term heat storage
for buildings.
2.5 Energy conversion efficiency
The process of converting water and air into
ammonia using electrolysis and the Haber-
Bosch process consumes energy. Similarly,
the cracking of ammonia to hydrogen also
consumes energy, as does the conversion
of hydrogen to electricity in a fuel cell. It
is possible to calculate all these losses
and express them as a percentage overall
efficiency – a measure of the energy output
compared to the energy input. In general, the
greater the number of processes involved,
the lower the overall efficiency, although
this depends upon the efficiency of those
processes. Overall efficiencies for different
ammonia uses are shown in Table 2, along with
hydrogen for comparison. The efficiency of
application becomes the main consideration,
if cheap green ammonia becomes an
internationally traded energy commodity.
Process Efficiency of ammonia
or hydrogen production
(renewable power from wind & solar)
Efficiency of
application
Overall
efficiency
Ammonia from electrolysis and
Haber-Bosch, used with a solid oxide
fuel cell to produce electricity
55 to 60% 50 to 65% 28 to 39%
Ammonia from electrolysis and
Haber-Bosch burned in an internal
combustion engine
55 to 60% 30 to 40% 17 to 24%
Hydrogen cracked from ammonia
obtained by electrolysis and Haber-
Bosch, and used in a PEM fuel cell
40 to 50% 40 to 50% 15 to 25%
Hydrogen from electrolysis and used
in a PEM fuel cell
65 to 70% 40 to 50% 26 to 35%
Modelled efficiencies for energy provided from primary electricity
49
.
TABLE 2
32 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

CHAPTER TWO
CASE STUDY 4
Decarbonising the international maritime sector
The International Maritime Organisation has committed
to reducing greenhouse gas (GHG) emissions from
international shipping by at least 50% (compared to
2008) by 2050. One of the key challenges in achieving
these targets is the longlifetime oflarge ships (around 25
years).
The maritime industry has already identified the
significant retrofitting potential for ammonia as a green
fuel for shipping, noting its ease of storage, existing
maritime networks and bunkering capabilities, flexible
use in both combustion engines and fuel cells and
potential relative to other decarbonisation options
50
.
A recent Environmental Defence Fund (EDF) report
discusses the decarbonising potential of ammonia in
theinternational maritime sector and highlights Morocco,
which is already investing in large-scale solar energy
generation, as a potential key player with large commercial
ports close to key shipping routes and an abundance
of renewable energy resources
51
. These include a total
potential for offshore wind of around 250GW, which is
approximately 25 times the current generating capacity
in the country and would provide 770TWh of electricity
annually, which is sufficient to produce green ammonia
forabout a third of the international shipping fleet.
MAN Energy Solutions, a designer and manufacturer
ofmarine engines, have committed to decarbonising
the maritime economy starting with fuel decarbonisation
in container shipping. They are currently developing
ammonia fuelled-engines based on current liquid natural
gas technology and anticipate that the first ammonia
engine could be in operation by early 2022
52
. MAN
Energy Solutions is also in the process of obtaining flag
state approval to use ammonia as a marine fuel in the
IGC Code (International Code of the Construction and
Equipment of Ships Carrying Liquefied Gases in Bulk).
The accredited classification society, DNV-GL, with 24%
of the market share in shipping, arealso pursuing the
use of ammonia as a marine fuel. Other developments
include Lloyd’s Register granting Approval in Principle
to SDARI (Shanghai Merchant Ship Design& Research
Institute) for the design of a 180,000 ton ammonia-
fuelled bulk carrier
53
and announcing a project for an
ammonia-fuelled 23,000 TEU ultra-large container
ship (ULCS) concept design fromMAN-ES and DSIC
(Dalian Shipbuilding Industry Co)
54
. Furthermore ABS
(American Bureau of Shipping), MAN-ES andSDARI
are collaborating to develop ammonia-fuelled
feedervessels
55
.
50. Gong W, Willi ML. 2008 United States Patent Application Publication – Caterpillar Inc. See https://patentimages.storage.googleapis.com/b4/
b0/74/315157b86c9292/US20100019506A1.pdf (accessed 14 November 2019).
51. Ash N, Scarbrough T. 2019 Sailing on Solar: Could green ammonia decarbonise international shipping? Environmental Defense Fund.
See https://europe.edf.org/file/399/download?token=agUEbKeQ (accessed 23 May 2019).
52. MAN Energy Solutions. 2019 Engineering the future two-stroke green-ammonia engine. See https://marine.man-es.com/docs/librariesprovider6/test/
engineering-the-future-two-stroke-green-ammonia-engine.pdf?sfvrsn=7f4dca2_4 (accessed 14 November 2019).
53. Shanghai Merchant Ship Design & Research Institute. 2019 Linkedin https://www.linkedin.com/posts/shanghai-merchant-ship-design-%26-research-
institute_180k-dwt-bc-of-carbon-free-issued-and-obtained-activity-6609776461731717120-oZtk/ (accessed 20th December 2019).
54. Lloyd’s Register. 2019 Industry project to design ammonia-fuelled 23k ULCS concept. See https://www.lr.org/en/latest-news/aip-ammonia-fuelled-ulcs/
(accessed 20 December 2019).
55. American Bureau of Shipping (ABS). 2019 ABS, MAN & SDARI join forces to develop ammonia-fuelled feeder vessel. See https://ww2.eagle.org/en/
news/press-room/abs-man-sdari-develop-ammonia-fueled-feeder-vessel.html (accessed 20 December 2019).
TEU – Twenty-foot equivalent units.
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 33

CHAPTER THREE
34 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE
International perspectives:
activities and future opportunities
There is significant future export potential for
stored renewable energy
56
. Indeed, the ability
to store and transport sustainable energy
worldwide may be one of the cornerstones
of a zero-carbon energy future. First plans
for the international trading of ammonia as
a renewable energy commodity involve
rich, solar- and wind-resourced countries
and regions. The UK has an excellent
source of renewable wind energy and has
the technological know-how to be a world
leader in the development and use of green
ammonia. Developments in three other
countries are highlighted here to demonstrate
the global effort in green ammonia.
3.1 Japan
In 2015, the Japanese government launched
the R&D programme Strategic Innovation
Promotion Program – Energy Carriers, which
focused on the entire hydrogen energy
value chain, from production, through
transportation and storage, to consumption.
These technologies will be demonstrated
at the Tokyo Olympics in 2020. Part of the
programme explored the potential routes for
importing significant quantities of hydrogen-
containing materials produced in locations with
abundant renewable energy potential such
as Australia and the Middle East. The energy
storage methods under investigation were
liquid hydrogen, liquid organic hydrides (with
primary focus on methyl cyclohexane) and
ammonia . As a result of the Energy Carriers
demonstrations, the Ministry of Energy, Trade,
and Industry added ammonia to its latest
technology roadmap. This has been signed
into law and the new Hydrogen Basic Strategy
has called for imports of carbon-free ammonia
“by the mid-2020s”
57
.
Low levels of ammonia in co-firing with
coal and natural gas have demonstrated
stable combustion in Poland and Japan,
while companies such as IHI (Japan) have
announced initial trials to supply power up to
2MW in one of their coal-converted units, with
a goal to replace more than 20% of coal for
the production of cleaner power
58
. Similarly,
the chemical company, Ube Industries Ltd.
has successfully replaced coal with ammonia
in initial tests for clinker production, and have
found that the quality and strength of the final
product remained the same.
Research is also underway which demonstrates
the feasibility of ammonia:hydrogen blends
for burning in gas turbines
59
. This indicates
ammonia has the potential for use in combined
cycle gas turbines (CCGT), which provide a
high degree of flexibility in meeting electricity
demand and compensating for the variability
in renewable electricity from wind and
solarsources.
56. Cross-ministerial Strategic Innovation Promotion Program (SIP). 2015 Energy Carriers.
See http://www.jst.go.jp/sip/pdf/SIP_energycarriers2015_en.pdf (accessed 14 October 2019).
57. Ministerial Council on Renewable Energy Hydrogen and Related Issues. 2017 Basic Hydrogen Strategy.
See https://www.meti.go.jp/english/press/2017/pdf/1226_003b.pdf (accessed 14 October 2019).
58. IHI Corporation. 2018 World’s highest level of combustion test facilities for coal-fired power plants.
See https://www.ihi.co.jp/ihi/all_news/2017/technology/2018-3-28/index.html (accessed 14 October 2019).
59. Kobayashi H, Hayakawa A, Somarathne KDKA, Okafor EC. 2019 Science and technology of ammonia combustion.
Proceedings of the Combustion Institute, 37, 109-133. (doi: 10.1016/j.proci.2018.09.029).

CHAPTER THREE
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 35
3.2 Australia
Given its significant potential for large-scale,
low-cost renewable electricity, Australian
federal and state governments and the
Commonwealth Scientific and Industrial
Research Organisation (CSIRO) have recently
published several hydrogen roadmap
documents, which lay out a trajectory to large-
scale production and export of hydrogen from
Australia. These plans include the synthesis
of green ammonia
60
. In total, the Australian
Renewable Energy Agency (ARENA) has
granted A$2.9million (approx. £1.5million) to
deliver feasibility studies at two ammonia
plants in Queensland. Firstly, A$1.9million
(approx. £1million) worth of federal grants
was provided to Queensland Nitrates Pty
Ltd (QNP) to fund a technical and economic
feasibility study that will assess the production
of renewable ammonia at a commercial
scale using an existing plant in Central
Queensland. The aim is to produce 20,000
tonnes of ammonia per year (around 20% of
Queensland’s nitrate demand)
61
. The second
plant is in Moranbah, Queensland, where
around A$1million (approx. £520,000) was
awarded to Dyno Nobel to conduct a similar
feasibility study. Both studies aim to identify
methods to accelerate the development of
industrial-scale electrolysis equipment and
help lower the costs.
In Western Australia, another recent example
is the feasibility study by ENGIE SA and YARA
International ASA to design a green hydrogen
plant that would be integrated with an existing
YARA ammonia plant in Pilbara
62
.
CSIRO has been developing membrane
technology to produce pure hydrogen streams
from cracked ammonia suitable for refuelling
PEM fuel cell vehicles
63
. This technology
was demonstrated in 2018 and is currently
incommercial development.
3.3 China
Recent International Energy Agency
analyses have explored the potential to
significantly reduce the cost of renewably-
produced hydrogen and ammonia by using
a combination of wind and solar resources in
areas of East China
64
. The analysis showed
that by using these renewable energy
resources, cost reductions in the region of
10 – 20% could be achieved, particularly if
the Haber-Bosch process could be run with
increased flexibility than current modes of
operation. If realised, the estimated lowest
cost of ammonia values of between £380
– 420/tonne are close to being competitive
withcoal-based ammonia production in the
region even without CCS costs added.
60. Bruce S et al. 2018 National Hydrogen Roadmap. CSIRO, Australia.
61. Australian Renewable Energy Agency. 2019 Queensland green ammonia plant could use renewable hydrogen.
See https://arena.gov.au/news/queensland-green-ammonia-plant-could-use-renewable-hydrogen/
(accessed 14 November 2019).
62. ENGIE. 2019 ENGIE and YARA take green hydrogen into the factory. See https://www.engie.com/en/news/yara-green-
hydrogen-factory/ (accessed 14 November 2019).
63. Lamb KE et al. 2018 High-Purity H2 Produced from NH3 via a Ruthenium-Based Decomposition Catalyst and
Vanadium-Based Membrane. Ind. Eng. Chem. Res, 57, 8–13. (doi: 10.1021/acs.iecr.8b01476).
64. International Energy Agency. 2019 The Future of Hydrogen. See https://www.iea.org/hydrogen2019/
(accessed 29 May 2019).

Conclusion
The global production of 176 million tonnes of
ammonia per year accounts for around 1.8%
of overall global carbon dioxide emissions.
To meet net-zero targets, an urgent plan to
decarbonise ammonia production must be
developed and implemented, which in turn
would open opportunities for ammonia to
replace fossil fuels in other applications.
The majority of the carbon dioxide emitted during
ammonia production comes from thesteam
methane reforming (SMR) process for hydrogen
production. In the short-term, to manage the
transition to net-zero carbon systems, ‘blue
hydrogen’ can be produced by incorporating
carbon capture and storagealongside the
SMR process. Thisisunlikely to be a long-term
solution inazero-carbon economy.
The electrolysis of water to produce ‘green
hydrogen’ offers a pathway to zero-carbon
ammonia production but relies on low-
cost sustainable electricity and continuing
reductions in electrolyser costs. Renewable
energy electricity costs from regions rich in
wind and solar energy (at prices between 1.7
and 3.4 GBP pence/kWh) are already close to
a tipping point for the affordable production
of zero-carbon green ammonia. The value of
a green ammonia market would significantly
strengthen the economic opportunities to
extend renewable penetration into the energy
economy. However, while the overall efficiency
remains poor, the energy system must be
considered to ensure that production of
ammonia is relevant to the local situation.
There are several processes that could be
developed with further research to produce
‘green ammonia’ that include new production
catalysts, electrochemical ammonia production
and chemical looping processes. Some
of these technologies may address the
challenges of directly coupling ammonia
production to intermittent renewable power.
In addition to decarbonising the existing
uses of ammonia, such as the production of
fertilisers for agriculture, the production of
green ammonia from green hydrogen could
offer further options in the drive to reduce
greenhouse gas emissions:
• As an energy storage medium, ammonia
is easily stored in large quantities as a
liquid at modest pressures (10 – 15 bar) or
refrigerated to -33°C. In this form, its energy
density is around 40% that ofpetroleum.
• As a zero-carbon fuel, can also be used
in fuel cells or by combustion in internal
combustion engines, industrial burners and
gas turbines. The maritime industry is likely
to be an early adopter of ammonia as a fuel.
Ammonia also has the potential to be used
to decarbonise rail, heavy road transport
and aviation.
• To generate electricity through fuel cells,
gas turbines or international combustion
engines to provide power to the grid or
remote locations.
• As an effective energy carrier for nascent
international sustainable energy supply
chains. It is lower cost and significantly easier
to store and transport than pure hydrogen,
has existing international infrastructure, can be
cracked to produce hydrogen when required
and is itself a zero-carbon fuel.
• Has the potential to be used in district
heating systems.
A global manufacturing and distribution system
is in place. While the safe transportation and use
of ammonia is well-established, new applications
will require careful risk assessment and
additional control measures may be required to
reduce risks to health and the environment.
The UK possesses expertise in catalysis,
combustion, fuel-cell technologies and
electrolysis that will be key to improving the
efficiencies and reducing the costs of ammonia
combustion, low-carbon hydrogen production,
ammonia cracking and the development of a
broad range of fuel cell technologies.
CONCLUSION
36 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE

ANNEX
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 37
Annex A: Definitions
Technology readiness levels (TRL)
Technology Readiness Levels (TRLs) are a
technology management tool that provides
ameasurement to assess the maturity of
evolving technology.
Units used in the report.
In the International System of units (SI), energy
is measured in joules (J). Power is the rate of
energy used and is measured in joules per
second or watts (W).
The SI system uses the following scale prefixes:
To simplify the numbers (eg for domestic
energy bills), energy is sometimes quoted
in watt hours (Wh), which is the amount of
energyused at a rate of one watt for one
hour= 3,600 Joules.
For example: running a 1,000 watt (1 kW)
heater for 1 hour has used 1,000 Wh or 1 kWh
of energy (if expressed in joules this would be
3,600,000 J or 3.6 MJ)
Energy content is often expressed in multiples
of watt hours eg MWh, GWh, TWh.
Rate of production given in multiples ofwatts
eg MW, GW.
Btu = The British thermal unit is a non-SI,
traditional unit of heat. MMBtu is 1,000,000Btu
and is equivalent to 293kWh.
Currency exchange (as of 28 November 2019)
System Technology
readiness
level (TRL)
Hydrogen production from ammonia
Ammonia cracking (>700°C) 7 – 9
Ammonia cracking (~450°C)
(non-precious metal)
2 – 4
Ammonia purification 3 – 6
Ammonia production methods
Decarbonised Haber Bosch 5 – 9
Chemical looping processes 1 – 4
Electrochemical production 1 – 3
Biological ‘nitrogen fixation’
production
1
R&D: decarbonised Haber Bosch production
Improving Haber Bosch
catalysts (lower temperature)
1 – 4
Ammonia separation methods
(lower-pressure operation)
1 – 5
Number Name Symbol
1,000 Kilo k
1,000,000 Mega M
1,000,000,000 Giga G
1,000,000,000,000 Tera T
1,000,000,000,000,000 Peta P
1USD 0.774GBP
1EUR 0.852GBP
1AUD 0.524GBP
Basic
technology
research
Research
to prove
feasibility
Technology
development
Technology
demonstration
System /
subsystem
development
System test,
launch and
operations
TRL 1
TRL 2
TRL 3
TRL 4
TRL 5
TRL 6
TRL 7
TRL 8
TRL 9

ANNEX
38 AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE
Annex B: Acknowledgements
This policy briefing is based on discussions from a workshop held at the Royal Society on 8th
June 2018 and subsequent input. The Royal Society would like to acknowledge the contributions
from those people who attended the workshop, and helped draft and review the policy briefing.
Chair project leader
Professor Bill David FRS, Professor of Chemistry, STFC Senior Fellow, University of Oxford
Steering group
Professor Fraser Armstrong FRS, Department of Chemistry, University of Oxford
Professor Phil Bowen, School of Engineering, Cardiff University
Professor David Fowler FRS, Centre for Ecology and Hydrology
Professor John Irvine, School of Chemistry, University of St Andrews
Dr Laura Torrente Murciano, Department of Chemical Engineering and Biotechnology,
University of Cambridge
Policy briefing contributors
Ms Debbie Baker, CF Fertilisers
Mr Trevor Brown, Consultant, Ammonia Energy Association
Mr Nicholas Cook, CF Fertilisers
Professor Sir Steve Cowley FREng FRS, Princeton Plasma Physics Laboratory
Mr Stephen Crolius, Consultant, Ammonia Energy Association
Professor Sir Chris Llewellyn Smith FRS, Department of Physics, University of Oxford
Dr Josh Makepeace, School of Chemistry, University of Birmingham
Dr Cédric Philibert, Senior Analyst, International Energy Agency
Dr Agustin Valera-Medina, Cardiff University
Dr Ian Wilkinson, Programme Manager, Siemens
Dr Tom Wood, Science and Technology Facilities Council

ANNEX
AMMONIA: ZEROCARBON FERTILISER, FUEL AND ENERGY STORE 39
Workshop attendees
Professor René Bañares-Alcántara, Department of Engineering Science, University of Oxford
Mr Chris Bronsdon, Director, Eneus Energy
Ms Morna Cannon, Department for Transport
Professor Richard Catlow FRS, Department of Chemistry, University College London
Mr Phil Cohen, Department for Business, Energy and Industrial Strategy
Dr Sam French, Senior Business Development Manager, Johnson Matthey
Professor Brian Foster FRS, Department of Physics, University of Oxford
Dr Francisco Garcia Garcia, School of Engineering, University of Edinburgh
Dr John Hansen, Senior Principal Scientist, Haldor Topsøe
Professor Justin Hargreaves, School of Chemistry, University of Glasgow
Professor Tim Mays, Institute for Sustainable Energy and the Environment, University of Bath
Mr Richard Nayak-Luke, Department of Engineering Science, University of Oxford
Dr Thoa Thi Minh Nguyen, Haldor Topsøe
Dr Andy Pearson, Group Managing Director, Star Technical Solutions
Mr Samir Prakash, Head of Emerging Technologies, Government Office for Science
Dr Carlo Raucci, Principal Consultant, U-MAS
Dr Ronan Stephan, Scientific Director, Plastic Omnium
Dr Rob Stevens, Vice President – Decarbonise Technology, Yara International
Ms Rita Wadey, Deputy Director, Department for Business, Energy and Industrial Strategy
Dr Chris Williams, Manager- Energy Research, Tata Steel
Royal Society Staff
The Royal Society would also like to acknowledge the contributions from the following members
of staff in creating this policy briefing:
Royal Society Staff
Frances Bird, Policy Adviser, Resilient Futures
Alex Clarke, Policy Adviser, Resilient Futures
Paul Davies, Senior Policy Adviser, Resilient Futures
Elizabeth Surkovic, Head of Policy, Resilient Futures

ISBN: 978-1-78252-448-9
Issued: February 2020 DES5711
The Royal Society is a self-governing Fellowship of many
of the world’s most distinguished scientists drawn from all
areas of science, engineering, and medicine. The Society’s
fundamental purpose, as it has been since its foundation
in 1660, is to recognise, promote, and support excellence
in science and to encourage the development and use of
science for the benefit of humanity.
The Society’s strategic priorities emphasise its commitment
to the highest quality science, to curiosity-driven research,
and to the development and use of science for the benefit
of society. These priorities are:
• Promoting excellence in science
• Supporting international collaboration
• Demonstrating the importance of science to everyone
For further information
The Royal Society
6 – 9 Carlton House Terrace
London SW1Y 5AG
T +44 20 7451 2500
E science.policy@royalsociety.org
W royalsociety.org
Registered Charity No 207043
9 781782 524489
