
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 1 of 149
VHA Office of Research and Development
Manual for Administrative Officers and
Associate Chiefs of Staff
Version 3.5
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 2 of 149
TABLE OF CONTENTS
FOREWORD ................................................................................................................................................... 8
INTRODUCTION ............................................................................................................................................. 9
FINDING HELP ...................................................................................................................................... 10
ORD RESEARCH – Office of Research & Development ................................................................................ 11
An Overview of the Organization and Structure of ORD ..................................................................... 11
Actively Manage Portfolios and Priority Areas of Research ................................................................ 13
Who’s Who in Central Office ................................................................................................................... 14
Who’s at Your Facility .............................................................................................................................. 14
Field Research Advisory Committee ........................................................................................................ 15
National Research Advisory Council ........................................................................................................ 15
Key Contacts ORD-Wide .......................................................................................................................... 16
VA Regions and VISN Area ....................................................................................................................... 17
Field Research Programs ............................................................................................................................. 18
THE ADMINISTRATIVE OFFICER ................................................................................................................... 19
THE ASSOCIATE CHIEF OF STAFF, RESEARCH & DEVELOPMENT .................................................................. 20
ACRONYMS .................................................................................................................................................. 21
SECTION 1 – Basic Information
................................
.................................................................................... 25
What is Research? ................................................................................................................................... 25
Why Does the VA have a Research Program? ......................................................................................... 25
VA and VHA Handbooks and Directives................................................................................................... 27
SECTION 2 – Getting a Research Project Approved at the “X” VA ............................................................... 28
How to Determine If a Project Meets the Definition of VA Research ..................................................... 28
Who Can Be a VA Investigator? ............................................................................................................... 28
The Process to Become a VA Investigator ............................................................................................... 28
Principal Investigator Orientation ........................................................................................................... 28
1. To initiate a project, contact the Research Office ....................................................................... 29
2. What subcommittee approvals are needed for the proposed project? ...................................... 30
3. R&D Approval .............................................................................................................................. 32
4. Required Training to Perform Research at the VA ...................................................................... 32
SECTION 3 – Finance Background ................................................................................................................ 33
Types of Money ....................................................................................................................................... 34
V
eterans Equitable Resource Allocation (VERA) Funds ....................................................................... 34
Award Funds ........................................................................................................................................ 36
Fina
ncial Systems .................................................................................................................................... 36
VistA .................................................................................................................................................... 36
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 3 of 149
RDIS ..................................................................................................................................................... 36
FMS ...................................................................................................................................................... 37
Available Resources ................................................................................................................................. 39
WinRMS ............................................................................................................................................... 39
RAFT – Research Analysis and Forecasting Tool .................................................................................. 39
Income ..................................................................................................................................................... 41
Expense ................................................................................................................................................... 42
Contracts – What are they and how are they funded? ....................................................................... 42
General Post Funds ................................................................................................................................. 44
Managing Research Funding ................................................................................................................... 45
VA Nonprofit Corporation ....................................................................................................................... 45
SECTION 4 – Human Resources (HR) ........................................................................................................... 47
Appointment Types ................................................................................................................................. 47
Appointment Authorities – Title 38 vs Title 5 .......................................................................................... 48
Appointment Authorities ........................................................................................................................ 48
Competitive Status .............................................................................................................................. 48
Noncompetitive and Excepted Appointments .................................................................................... 49
Schedule B ........................................................................................................................................... 51
Schedule A Appointments ................................
.................................................................................... 51
Title 38 and Title 38 Hybrids ................................................................................................................ 51
Veterans’ Appointments...................................................................................................................... 52
Use of Schedule B Vs. Title 38 Medical Support Authority .................................................................. 53
VA Recruitment Process in Brief .......................................................................................................... 53
Without Compensation Employees ......................................................................................................... 54
Intergovernmental Agency Personnel Agreements ................................................................................ 55
Regulatory Authorities ........................................................................................................................ 55
Which organizations can participate in the IPA program? .................................................................. 55
Which individuals can come to the VA on an IPA? .............................................................................. 56
Term Limits of IPAs .............................................................................................................................. 56
Setting Up an IPA ................................................................................................................................. 56
Joint Personnel Agreements (JPA) ........................................................................................................... 58
Factors to Consider When Hiring VA Employees in Research Service ..................................................... 58
Request for Personnel Action .................................................................................................................. 58
Recruitment ............................................................................................................................................. 59
Disciplinary Actions and Union Interaction .............................................................................................
59
On-
the-Job Injury .................................................................................................................................... 60
Performance Appraisals .......................................................................................................................... 60
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 4 of 149
Incentive Awards ..................................................................................................................................... 61
Quality Step Increase (QSI) ...................................................................................................................... 61
Managing Office Schedules and Leave .................................................................................................... 62
Liaison with HR ........................................................................................................................................ 62
Tips on Working with Human Resources ............................................................................................. 63
Sabbaticals - Local ................................................................................................................................... 63
SECTION 5 – Applying for VA-funding
eRA and Merit Review ................................................................... 64
Eligibility .................................................................................................................................................. 66
Calendar .................................................................................................................................................. 67
Submission Process ................................................................................................................................. 67
System for Award Management .............................................................................................................. 67
Pre-Review .............................................................................................................................................. 68
Award ...................................................................................................................................................... 68
Post Award .............................................................................................................................................. 68
ShEEP (Shared Equipment Evaluation Program) and LAMb (Laboratory Animal Major Equipment)
Program ................................................................................................................................................... 69
Protected Time for Research and Relationship to VERA ......................................................................... 69
Growing a Program – Career Development Award ............................................................................. 74
C
areer Scientist .................................................................................................................................... 75
Starting a New Program ...................................................................................................................... 76
SECTION 6 – Travel ...................................................................................................................................... 78
SECTION 7 – Working with Supply Chain Management .............................................................................. 83
Equipment Inventories ............................................................................................................................ 83
SECTION 8 – Credentialing for Research Staff Engaged In Human Subjects Research Projects at the
XVAMC ........................................................................................................................................................ 85
Types of Personnel Engaged in Human Subjects Research ..................................................................... 85
Responsibilities ........................................................................................................................................ 85
Research Scope of Practice or Scope of Work ......................................................................................... 85
Who Needs Clinical Privileging through VetPro? ..................................................................................... 86
VetPro .................................................................................................................................................. 86
Registered Nurses ............................................................................................................................... 86
Human Studies Orientation ................................................................................................................. 86
How to determine if someone is engaged (or not engaged) in human subjects research .................. 87
You are Not Engaged in human research if ......................................................................................... 87
SECTION 9 – VA Training Requirements
................................
...................................................................... 88
All Research Personnel Engaged in Human Subjects Research ............................................................... 88
Personnel Engaged in Animal Research .................................................................................................. 88
Personnel Engaged in Laboratory Research ............................................................................................ 88
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 5 of 149
Training Required of all Working in VA Research .................................................................................... 88
SECTION 10 – General Administrative Management .................................................................................. 89
Help Resolve Any Issues That Arise ..................................................................................................... 89
Committees ......................................................................................................................................... 89
Customer Service ................................................................................................................................. 90
Working with Clinical Services ................................................................................................................. 91
Research Space ........................................................................................................................................ 92
Assignment and Review of Space ........................................................................................................ 92
Renovations ......................................................................................................................................... 94
Tips in Space Management ................................................................................................................. 95
General Oversight of Research Lab Areas and Other Odds and Ends...................................................... 95
Enter Work Orders to Engineering ...................................................................................................... 95
Examples Engineering Work Orders .................................................................................................... 95
Involvement in Emergency Preparedness Plan ................................................................................... 95
Emergency Cascade Plan ..................................................................................................................... 96
Facility Level ........................................................................................................................................ 96
New Staff ............................................................................................................................................. 96
PIV B
adges ........................................................................................................................................... 96
Service Needs ...................................................................................................................................... 96
Oversight of Construction and Upgrade Projects ................................................................................ 97
Key Requests ....................................................................................................................................... 97
Hood Certification ............................................................................................................................... 97
Prescription Pads for Research MDs.................................................................................................... 97
Staff Meetings ..................................................................................................................................... 98
Researcher Communication and Meetings ......................................................................................... 98
Research Week Event Planning and Coordination .............................................................................. 98
SECTION 11 – Information Technology (IT) ................................................................................................. 99
IT Allocation – Attention to Source of Computers and Data Storage Devices ......................................... 99
VHA Research and Development IT/Informatics Guidance FAQs (Updated Annually) ............................. 101
ADPAC – Automated Data Package Application Coordinator ................................................................ 101
Information System Security Officer (ISSO) ........................................................................................... 101
Privacy Officer ....................................................................................................................................... 102
SECTION 12 – Project Management .......................................................................................................... 103
C
ompliance Issues .................................................................................................................................
103
Tr
acking Dates ....................................................................................................................................... 103
Liaison for Construction and Engineering Projects ................................................................................ 103
Paperwork Reduction Act ...................................................................................................................... 103
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 6 of 149
SECTION 13 – Compliance ......................................................................................................................... 104
ORO ....................................................................................................................................................... 104
RCO ........................................................................................................................................................ 105
Corrective Action Plan ........................................................................................................................... 105
SECTION 14 – Updating Databases ............................................................................................................ 106
ePROMISe .............................................................................................................................................. 106
RDIS – Accessed thru ePROMISe ............................................................................................................... 106
VAIRRS (VA Innovation and Research Review System) ............................................................................. 106
SECTION 15 – Affiliate University .............................................................................................................. 107
Time Effort MOU Guidance ................................................................................................................... 107
Background ........................................................................................................................................ 107
Issues ................................................................................................................................................. 108
Plan .................................................................................................................................................... 108
Guidance for MOUs ........................................................................................................................... 108
NIH Requirements for Time and Effort MOU .................................................................................... 109
SECTION 16 - VA Non-Profit Corporations ................................................................................................. 111
SECTION 17 – Local Facility ........................................................................................................................ 114
Services You’ll Interact With .............................................................................................................. 114
Em
ergency Procedures .......................................................................................................................... 115
SECTION 18 – Safety & Security ................................................................................................................ 116
Directives & Handbooks ........................................................................................................................ 116
SRS (Subcommittee on Research Safety) .............................................................................................. 116
Safety Compliance ................................................................................................................................. 116
Security .................................................................................................................................................. 116
Information System Security Officer (ISSO) ........................................................................................... 117
Police Service ......................................................................................................................................... 117
SECTION 19 – Privacy ................................................................................................................................ 118
Directives & Handbooks ........................................................................................................................ 118
SECTION 20 – Animal Program .................................................................................................................. 119
Handbooks............................................................................................................................................. 119
Veterinary Medical Unit ........................................................................................................................ 119
Institutional Animal Care and Use Committee ...................................................................................... 119
Assurance .............................................................................................................................................. 120
Re
ports .................................................................................................................................................. 120
SECTION 21 – Site Visit Coordination ........................................................................................................ 121
SECTION 22 – IBC Registration and Annual Reporting ...............................................................................
122
I
nstitutional Biosafety Committee ........................................................................................................ 122
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 7 of 149
Who Must Be On the IBC ................................................................................................................... 122
The Institution shall file an annual report with NIH/OBA, which includes ........................................ 122
SECTION 23 – Human Research Protection Program ................................................................................ 123
Office of Research Protections, Policy and Education ........................................................................... 123
FWA or Federal-Wide Assurance ........................................................................................................... 123
Belmont Report ..................................................................................................................................... 123
The Common Rule ................................................................................................................................. 124
Working with Boards and Committees .................................................................................................. 124
Research and Development Committee ........................................................................................... 124
Institutional Review Board (IRB) ........................................................................................................ 125
Affiliate IRB ........................................................................................................................................ 125
VA Central IRB ................................................................................................................................... 125
VA Central IRB Liaison ....................................................................................................................... 126
IRB of Another Federal Agency.......................................................................................................... 126
Single IRB Mandate (https://www.research.va.gov/programs/orppe/single_irb.cfm) ...................... 126
Subcommittee on Research Safety .................................................................................................... 126
Non-Research Committees Responsible for the Review of Research
................................
................... 127
Pharmacy and Therapeutics Committee ........................................................................................... 127
Radiation Safety Committee.............................................................................................................. 127
Key Issues to Address When Terminating Human Subject Studies at a VA Facility with a Human
Research Protection Program ............................................................................................................... 128
VHA Office of Research and Development, March 25, 2016 ............................................................. 128
SECTION 24 – SOPs .................................................................................................................................... 130
SECTION 25 – VA Technology Transfer Program (TTP) .............................................................................. 131
Cooperative Research and Development Agreements (CRADAs) ......................................................... 131
SECTION 26 – Records Control Schedule ................................................................................................... 133
Getting Started ...................................................................................................................................... 134
Policies, Resources, and Helpful Links ................................................................................................... 136
SECTION 27 – Financial Conflict of Interest The FCOI Form ..................................................................... 138
SECTION 28 – Publishing VA Research ...................................................................................................... 140
SECTION 29 – Communications Working with the Public Affairs Office and ORD Communications ........ 141
SECTION 30 – Research Misconduct Transgressions in Research ............................................................. 142
Appendix A – VHA DIRECTIVES AND HANDBOOKS and PROGRAM GUIDES
................................
.............. 144
A
ppendix B– Useful Web Links .................................................................................................................. 145
Appendix C – Research Calendar ............................................................................................................... 146
Appendix D– Other Commonly Used Forms .............................................................................................. 149
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 8 of 149
FOREWORD
This third iteration of the ACOS/AO Handbook updates many sections based on the numerous changes
in policy and procedures that have occurred over the past 3 years. We plan to continue updating every
two years or as needed.
Indeed, Research continues to be the best kept secret in the VA; however, many steps are underway to
maximize communication to the field and stakeholders. Included are 9 initiatives and a reorganization
that will optimize research over the next few years.
VA continues to support an intramural program that blends the Clinical Care mission of the VA with its
Research mission, with clinician scientists and non-clinician scientists seeking new and innovative ways
to improve clinical diagnosis and therapies that will benefit its Veteran patients. The VA’s Research
program addresses clinical issues directly affecting Veterans, however many of the discoveries made by
VA researchers are applicable to the population at large. In addition, VA research improves systems
processes in care delivery, engages Veterans, and helps ensure that they receive high-quality, high-value
health care. VA Research has been a key element in attracting and recruiting the best and brightest
clinicians and non-clinicians to its facilities, as well as a tool to retain these extraordinary individuals.
Underlying the research itself, and the investigators and staff who are directly engaged in the research,
are unheralded individuals and teams who comprise Research Administration, and make it possible to
execute the research mission. This starts in the Office of Research and Development, the Veterans
Health Administration, and the VA Central Office, but it is carried out by the local Research Office on a
day-to-day basis at the individual VA Medical Centers. The burden of responsibility in ensuring that
investigators can perform their research duties – in the most supportive of an environment possible –
lies with the Research Administrative Officer and the Associate Chief of Staff for Research and
Development in the field office.
Thus, this Manual is dedicated to all in Research Administration, who are the behind-the-scenes folks
who facilitate the VA research enterprise.
“BECAUSE OF YOU, IT WORKS!”
The AO/ACOS Training and Mentoring Group
Special thank you to Holly Birdsall, MD, Dean Yamaguchi, MD and Marisue Cody, PhD for getting the ball
rolling on the initial version back in 2016. We want to also thank the Research Administration Council,
the ACOSs, the AOs, the NPPO, the ROTC-D and the ORD program officials who contributed to the
rewrite of this manual.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 9 of 149
INTRODUCTION
Research Administration in VA has evolved over the past 10 years since the version of the AO Guide was
compiled and published in 2012. It is still a fact that in VA Research programs around the country, no
two Administrative Officers (AO) do the same things from one facility to the next. However, as the
Office of Research and
Development (ORD) moves to
an enterprise system and the
communication between
programs strengthen, we have
ongoing opportunities to share
knowledge to increase
efficiencies in managing our
programs. For that reason, we
continue to update this
ACOS/AO Guide which provides
a practical source of
information for those who
oversee VA research
administration at the local level.
Due to the positive reception of the last version, this version of the guide has been updated based on
the number of changes that have occurred in the last two years. We expect that the document will
continue to evolve in the coming years but hope that with the new additions, it will continue to be a
resource for ACOSs, AOs, and other involved in managing VA Research.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 10 of 149
FINDING HELP
The ORD webpage has a large amount of useful information, FAQs, templates, etc.
• “Services” tab has information on BLR&D, HSR&D, CSR&D, and RR&D.
• “Programs” tab has information on Tech Transfer, Nonprofits, Cooperative Studies Program
(CSP), Million Veteran Program (MVP), and Animal Research. Information on human research,
including the central IRB is under the ORPP&E-Human Research Program heading. An interactive
FAQ page has been setup to address questions regarding a variety of questions on the conduct
of research. ORD highly encourages research staff to use this tool first.
• “For Researchers” tab has information on funding, and various policy and guidance documents
of use to the VAMC research office and investigators.
• “About Us” has contact information for ORD. It also has a nationwide directory of ACOS, AO,
and other field research office staff. You can search by name or by site.
• There are listserv groups that allow you to ask you colleagues for advice/help
o AOs = [email protected]
o ACOS = [email protected]
o Both listservs are monitored by staff in ORD. Contact [email protected] to be
added to these listservs.
• V
HA Office of Research and Development ACOS/AO Mentoring Program
o The Office of Research and Development (ORD) recognizes the complexities of running a
research program, no matter the size. Associate Chiefs of Staff, Research Program
Coordinators and Administrative Officers are expected to have a broad range of skills
sets and knowledge on a myriad of topics. ORD has developed a program that focuses
on advising on how to address many of the issues that research offices deal with on a
day-to-day basis. The Mentoring program was developed in collaboration with field-
based personnel to provide education, training, and mentorship to research
administrators in the VA’s intramural research program. The goal of the Research
Mentoring Program will be accomplished in three parts: 1) provision of a manual for
AOs and ACOS/R&D that can be used as a guide to basic operations of field VA research
administration; 2) individualized training, mentoring, and guidance; 3) a continuing
education program to outline changes in VA policies and directives that affect research
operations and disseminate best practices and workable solutions to issues and
problems faced in VA research administration
.
o The program is designed to be customized to each facility requesting assistance. At the
request of the facility Director, Chief of Staff, ACOS or AO, the mentoring team will
address focused or broad areas. Ultimately, the goal is to develop a network and
relationships where programs can rely on each other for expertise, guidance, and
assistance.
o ORD has developed a Program Assistance Consultation and Education Review (PACER)
to bring expertise regarding research administrative matters to the site. The PACER is a
2.5-day visit at the request of the Medical Center Director and covers a variety of areas
from space to regulatory issues. For further information contact the Director, Field
Operations.
o Should a mentoring visit be the best mechanism of assisting a Research Office, contact
the Director of Field Operations, ORD
o Questions can be directed to Antonio Laracuente, MBA (Director of Field
Operations, ORD.
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 11 of 149
ORD RESEARCH – Office of Research & Development
An Overview of the Organization and Structure of ORD
The Office of Research and Development (ORD) (14RD), in the VA Central Office, is part of the Veterans
Health Administration (VHA). The other parts of the Department of Veterans Affairs are the Veterans
Benefits Administration (VBA) and National Cemetery Administration (NCA). The leader of ORD is the
Chief Research and Development Officer, or CRADO. Established in 1947, this Congressionally- mandated
research program is unique among other Federal research programs in several ways: 1) It is focused
entirely on Veterans’ needs; 2) It is an intramural research program (i.e., only VA employees are eligible
to conduct VA research); and 3) It is the only Federally funded research program that is directly tied to a
fully integrated health care system. The mission of VA Research is four-fold: 1) to improve Veterans'
health and well-being via basic, translational, clinical, health services, and rehabilitative research; 2) to
apply scientific knowledge to develop effective and innovative individualized care solutions for
Veterans; 3) to attract, train, and retain the highest-caliber investigators, and nurture their development
as leaders in their fields; and 4) to assure a culture of professionalism, collaboration, accountability, and
the highest regard for research volunteers' safety and privacy that ensures partnered information flow
to and advanced care for Veterans – and for all those who rely on the VA health care system.
Currently, ORD includes four Research Services with the primary responsibilities of handling the reviews
of projects and programs submitted for funding consideration, as well as items specifically related to
investigator needs. These include Biomedical Laboratory R&D; Clinical Sciences R&D; Rehabilitation R&D,
and Health Services R&D. Biomedical Laboratory R&D (BLR&D) supports pre-clinical research to
understand life processes from the molecular, genomic, and physiological level in regard to diseases
affecting Veterans. Clinical Science R&D (CSR&D) supports clinical trials and other research to determine
the feasibility or effectiveness of new treatments (e.g., drugs and devices), compare existing therapies,
and improve clinical practice and care. Rehabilitation R&D (RR&D) supports research to develop novel
approaches to restore Veterans with traumatic amputation, central nervous system injuries, loss of sight
or hearing, or other physical and cognitive impairment to full and productive lives. Health Services R&D
(HSR&D) supports research at the interface of health care systems, patients, and outcomes examining all
aspects of VA health care (e.g., quality, access, patient outcomes, and costs). HSR&D includes the VA
Quality Enhancement Research Initiative (QuERI) to continuously improve VA health care by
systematically implementing clinical research findings and evidence-based recommendations into
routine clinical practice. In addition to the services, two ORD maintains enterprise-level programs that
also support scientific (including investigator initiated) work. The Cooperative Studies Program (CSP) is
ORD’s flagship clinical research enterprise division specializing in multisite clinical trials and
epidemiological research on health issues of vital importance to Veterans. The Million Veteran Program
(MVP) is ORD’s most recent group that focuses on genomic research and maintains a national
infrastructure for biospecimens, data and analysis. Both programs work with the ORD services on
conducting highly innovative approaches to addressing problems for the VA healthcare system and
nation.
Program Managers (PMs) within each R&D Service guide the Scientific Merit Review Boards as to
assignment of proposals to specific Boards and carry each proposal of the Board through the electronic
review process via eRA Commons and post-review collation of summary statements back to the Principal
Investigators or PIs. Program Managers also are involved in other specified administrative areas, such as
Career Scientist Awards, Career Development Awards, and other research-specific awards (e.g.,
Middleton, Barnwell, etc.), as well as eligibility, appeals, and RFA development, among others. Program
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 12 of 149
Managers are also involved in specialty research areas, including TBI consortium and regenerative
medicine.
In addition to the Services, there are other crosscutting programs within ORD. These include the
Biosafety and Biosecurity Program, the Animal Research Program, , the Gulf War Program, the
Biorepository Brain Bank Program, the Office of Research Protections, Policy, and Education (ORPP&E)
housing the Central IRB, the Health Disparities and Minority Health Research Program under the
umbrella of HSR&D, and the Women’s Health Research Program. A newly created division is the
Partnered Research Program which facilitates external (e.g., with industry) partnerships with particular
emphases on multi-site clinical trials involving the VA non-profit corporations. There is also a liaison office
in ORD with the Department of Defense and other Federal governmental agencies that perform research
(e.g., the National Institutes of Health and the National Science Foundation). The VA’s Technology
Transfer Program is also housed in ORD, as is the VA Non-Profit Program Office.
ORD also has sections that are involved in overall finance and budget matters affecting all R&D services
and ORD, including human resources, bioinformatics, information security, and the central clearinghouse
for inquiries directed to ORD from the Secretary and Under Secretary, Deputy Under Secretary for
Health for Policy and Services, other offices within VA, private organizations, and
additional too-
numerous-to-count entities. The day-to-day functions of Little ORD are under the auspices
of the ORD
Operations Officer.
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 13 of 149
Actively Managed Portfolios and Priority Areas of Research
Targeted or priority areas of research focus on Veteran specific or “Veteran-centric” areas of health care,
not generally seen or dealt with on a broad scale in the private sector. ORD has developed 4 actively
managed portfolios: Suicide, Brain Health, Deployment Exposures and Precision Oncology.
In addition, VA continues to address focus areas that include those related to returning combat Veterans
(not necessarily in order of importance):
•
Traumatic Brain Injury
•
Post-Traumatic Stress Disorder
•
Tissue Damage involving large areas due to blasts and projectile injury
•
Spinal Cord Injury
•
Prosthetics and Sensory Loss
•
Military Occupational Exposures (e.g., Agent Orange and Middle East conflict exposures)
•
Suicide Risk and Prevention
•
Risky Behaviors including substance use disorders including tobacco, alcohol, prescription pain
medications (e.g., opioids), and non-prescription street drugs
•
Military Service, Post-Deployment and Veteran-related mental health disorders, cognitive
problems, and other behavioral issues. Many Veterans have dual diagnoses of substance abuse
and mental health disorders.
•
Women’s Health
•
Genomic and Personalized or Precision Medicine
•
Chronic Metabolic Diseases and Syndromes, such as diabetes mellitus and pain, as well as the
use of Complementary and Alternative Medicine approaches for treatment of these conditions
•
Health Promotion and Disease Management
•
Pain Management
•
Access, Care Coordination, and Patient Centered Care
•
Airborne Hazards & Open Burn Pits
ORD interfaces with many parts of VA Central Office, but two partners are particularly important:
Office of Research Oversight (ORO) ensures the responsible conduct of VA research. ORO provides
oversight of compliance with VA and other Federal requirements for the protection of human research
subjects, laboratory animal welfare, research safety, research laboratory security, research information
security, and research misconduct. ORO also provides training to facility Research Compliance Officers
(RCO) and oversight of RCO auditing programs.
Office of Academic Affiliations (OAA) oversees VA’s teaching mission, its training programs, and its
affiliations with academic institutions.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 14 of 149
Who’s Who in Central Office
Who’s at Your Facility
• Medical Center Director (MCD) – The facility director is the CEO of the Medical Center. They
are also the Institutional Official for research.
• Deputy or Associate Director – Oversees Operations/Security/HR/ Labor – oversees all of the
day-to-day operational, employment, HR, and physical plant issues, and industrial hygiene that
support patient care services.
• Chief of Staff (COS) – Oversees the clinical policies, daily service issues that directly impact on
patient care: safety, census, emergencies and crisis management, appointment, and
credentialing of all clinicians. The ACOS/R&D generally reports directly to the COS.
• Chief, Nursing Service, Nurse Executive, or Associate Director for Patient Care Services –
Oversees hospital operations as it pertains to nursing staff/patient care and care management.
• Research Compliance Officer (RCO) – The RCO reports to the Facility Director and audits study
regulatory materials for compliance, as well as research office components for compliance.
• Chief of Research or Associate Chief of Staff/Research and Development (ACOS/R&D) – The
ACOS/R&D heads the research program at the facility, and helps in the recruiting, training, and
development of research investigators.
• Associate Chief of Staff Education (ACOS/E) – Manages educational and training mission for
health professions trainees of all disciplines and manages the academic affiliation.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 15 of 149
• Committee Manager – Manages committee activity, including agendas, minutes, rosters, filing
of protocol documentation, training of committee members, and follow-up tasks.
• Budget Analyst – A budget analyst manages money sent from ORD for research and
administrative use, ensuring that it is properly spent, and that none is left when the funding
expires.
• Procurement Technician – Orders supplies for research and research administration.
• Information System Security Officer (ISSO) – Oversees data security at the VA facility, tells you
how to protect information.
• Privacy Officer (PO) – Privacy of information, tells you what information needs to be protected,
and at some facilities is responsible for managing FOIA requests.
• Radiation Safety Officer – Liaison between Research Service and the Medical Center’s Radiation
Safety Program and the Radiation Safety Committee regarding all aspects of use of
radioisotopes and radiation producing machines in research.
Depending on the size of the research program there may be several other research administration
members, such as a Deputy ACOS for Research, Human Research Program Protection Administrator, a
Research Biosafety Officer, an Animal Program Officer, a Grants Administrator, and various Research
Committee managers.
Field Research Advisory Committee
In 2003, the Deputy Undersecretary for Health determined that the establishment of the Field Research
Advisory Committee (FRAC) was in the best interest of the VA research program. The purpose of the
Department of Veterans Affairs FRAC is to promote communication between ORD, Research Offices, and
investigators in the field, provide input and advice on issues relevant to VA research including current
operations, and participate in strategic planning. Membership is comprised of both VA Central Office
and field staff. More information can be found at
https://www.research.va.gov/resources/frac/default.cfm
National Research Advisory Council
The Council provides advice to the Secretary and the Undersecretary for Health (USH) and makes
recommendations on the nature and scope of research and development sponsored and/or conducted
by VHA to include: 1) the policies and projects of ORD; 2) the focus of research on the high priority
health care needs of Veterans; 3) the balance of basic, applied, and outcomes research; 4) the scientific
merit review process; 5) the appropriate mechanisms by which ORD can leverage its resources to
enhance the research financial base; 6) the rapid response to changing healthcare needs, while
maintaining the stability of the research infrastructure; and 7) the protection of human subjects in
research. More information can be found at https://www.va.gov/advisory/nrac.asp
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 16 of 149
Key Contacts ORD-Wide
ORD Central Office Contacts
https://www.research.va.gov/resources/ORD_Admin/ord_cont
acts.cfm
Central IRB
https://www.research.va.gov/programs/orppe/vacentralirb/def
ault.cfm
Cooperative Studies Program
https://www.research.va.gov/programs/csp/contacts.cfm
CTAA, CRADA, NPC/TTP issues,
Technology Transfer
https://www.research.va.gov/programs/tech_transfer/contacts
.cfm
ePROMISe Login
https://epromise.research.va.gov/epromise/login.htm
eRA Commons
https://public.era.nih.gov/commons/public/login.do?TARGET=
https%3A%2F%2Fpublic.era.nih.gov%2Fcommons%2Fcommons
Init.do
Financial Conflicts of Interest
OGCNorthAtlanticEthics@va.gov
OGCContinentalEthics@va.gov
OGCSouthEastEthics@va.gov
OGCPacificEthics@va.gov
Genomic Medicine Program (MVP)
https://www.research.va.gov/MVP/default.cfm
Human Subject and IRB Issues
(ORPP&E)
https://www.research.va.gov/programs/orppe/default.cfm
Just-In-Time (JIT)
https://vaww.gateway.research.va.gov/jit/
Nonprofit Program Office
https://www.research.va.gov/programs/nppo/default.cfm
Regulatory – Biosafety and General
VHACOORDRegulatory@va.gov
Specialty Team Advising Research
(STAR) VA OGC
https://vaww.ogc.vaco.portal.va.gov/law/research/default.aspx
Funding Information including RFAs
https://www.research.va.gov/funding/default.cfm
Biomedical Laboratory Research and
Development
https://www.research.va.gov/services/blrd/default.cfm
Clinical Science Research and
Development
https://www.research.va.gov/services/csrd/default.cfm
Health Services Research and
Development
https://www.research.va.gov/services/hsrd.cfm
Rehabilitation Research and
Development
https://www.research.va.gov/services/rrd.cfm

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 17 of 149
VA Regions and VISN Area
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 18 of 149
Field Research Programs
The most obvious reason for the differentiation in staffing at different facilities is the number of projects
and funding conducted by the center’s research program. It is useful to understand that research
programs at VA Medical Centers vary in size, complexity, and affiliation. For some large research
programs, there are centers, collaborations with different programs offices such as Rural Health and
infrastructure such as a large biomedical laboratory footprint.:
At facilities with a large research program, the AO leads a varied group of personnel that includes
budget, committees/regulatory, HR, and infrastructure support. The AO is equivalent to a Director of
Operations (or, perhaps, the conductor of an extremely diverse orchestra).
At facilities with smaller research programs with less overall resources, the AO must be a jack-of-all-
trades, and does many different tasks to support a smaller number of research projects. No matter
whether there are 10 projects or 500 projects, the AO must have a fundamental understanding of all
aspects of research management.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 19 of 149
THE ADMINISTRATIVE OFFICER
Administrative Officers (AOs) come in all shapes,
sizes, ages, and experiences. But AOs often share
some common characteristics. They are:
Organized. In all cases, an AO needs to be able to be
very organized and have systems in place to track the
myriad of tasks that need to be performed.
Connected. In talking to AOs from around the
country, one of the things heard most of the time is: you do not need to have all the answers, but you
do want to know who to contact regarding the questions that come up. AOs work closely with
committees, staff, researchers, and people in facility services to build consensus and create collective
solutions. Forming excellent working relationships with key individuals in other Medical Center Services
goes a long way to being successful in furthering the Research Mission. Sometimes those key individuals
are not the Service Chiefs, but front-line individuals who are facile in the actual day-to-day tasks.
Attentive. Many AOs are very good at attending to details, while being able to keep the bigger picture in
mind. These are individuals who can listen to the complaints or suggestions of a mega-funded
researcher, or a staff member, with equal focus.
Creative. AOs must often work with diverse groups and individuals and come up with unusual solutions.
Keep in mind, Principal Investigators (PIs) are people who need to think “outside the box” to find that
new understanding of an issue that no one had before. They are inherently creative and
entrepreneurial. So, if you are “stuck” on something, consult a PI – you might be surprised at the
solution they come up with for you.
Reasonable. We live in a world of regulations. You, ORO, accrediting organizations, and ORD may have
differing opinions on the correct interpretation of the regulations. You can be paralyzed by this
situation, or you can adopt a reasonable attitude and pursue the course of action that makes the most
sense to your group of decision-makers.
Patient. Most everyone you will work with has another job (or jobs). You may have to wait your turn to
get the attention that you need to resolve an issue.
Informed. As stagnant as people believe the government to be, its environment is in constant change.
Inform yourself as much as possible about new regulations, changes in staffing at your immediate
facility, changes in ORD funding focuses, key initiatives being pursued at your facility, as well as changes
going on at the affiliate university. As mentioned above, become familiar with whom to contact when
issues or questions arise.
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 20 of 149
THE ASSOCIATE CHIEF OF STAFF, RESEARCH & DEVELOPMENT
The Associate Chief of Staff, Research & Development (ACOS/R&D) is the Service Chief for Research
Service at VA Medical Centers (VAMC) that have a medium to large research program. This is generally a
full-time position, and usually paid from Medical Care funds. Currently, the ACOS/R&D can be either a
clinician or non-clinician PhD (with pre-approval from ORD). At a VAMC with a small program, the head
of the Research Service is usually called a Coordinator for Research and Development (C/R&D), and this
position can be a collateral duty position. At one time, the Research and Education Chief position was a
merged Chief of Staff (COS) for Research & Education, but this combined position no longer exists at
VAMCs. The ACOS/R&D reports to the COS.
The ACOS/R&D has several defined duties stipulated by Directive and Handbook, including:
• Serves as the Executive Secretary of the Research & Development Committee (R&DC) as a non-
voting member.
• Notifies a Principal Investigator in writing after the R&DC and all relevant subcommittees have
approved a study and that the study can be initiated.
• Ensures that research Without Compensation (WOC) appointments are appropriately justified
and the appointments comply with all applicable research, Human Resources Management, and
other VA policies.
The ACOS/R&D can also serve as a pre-reviewer of VA award submissions, or investigators may seek
advice from the ACOS/R&D in interpreting reviewer comments from critiques received on submitted
applications. The ACOS/R&D additionally serves as a liaison with ORD Program Managers on matters
that arise with the review process or other issues raised by the Merit Review Boards. For instance, the
ACOS/R&D would weigh in on potential appeals to Merit Review Board reviews and can not only advise
the Investigator if an appeal is warranted but would be the Point of Contact with ORD on any appeal
process. The ACOS/R&D also serves as the Point of Contact on Intellectual Property submission to the
VA Technology Transfer office and as a signatory on the VA Certification form needed for the Invention
Disclosure submission. The ACOS/R&D is usually the liaison between a university affiliate’s Intellectual
Property Office when inventors are dual appointees. Thus, the ACOS/R&D should possess similar
qualities outlined above regarding the AO and listening skills, patience, persistence, accepting criticism
with a “thick skin,” collegiality with both investigators and VA support services and personnel, and
tactfulness are qualities that are good to cultivate as an ACOS/R&D. Remember, while each individual
who is trying to get access to the AO or ACOS/R&D believes that his or her issue is paramount and
should rise to the top, the ACOS/R&D must be a good “prioritizer.” This means to make every individual
feel special, and to, above all, be fair. Try to keep in mind that the Research Office exists because of PIs
who garner both intramural and extramural funding and enhance the reputation of the VAMC.
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 21 of 149
ACRONYMS
A VA document would not be complete without a listing of acronyms.
14RD
Office of Research and Development
AAALAC
Association for Assessment and Accreditation of Laboratory Animal Care
AAHRPP
Association for the Accreditation of Human Research Protection Programs
ACES
Attendance and Cost Estimation System – (VHA External Conference Request System)
ACORP
Animal Component of Research Protocol
ACOS
Associate Chief of Staff
AO
Administrative Officer
AWE
Annual Workplace Evaluation
BLR&D
Biomedical Laboratory Research and Development
CDW
Corporate Data Warehouse
CFR
Code of Federal Regulations
CITI
Collaborative Institutional Training Initiative – (training modules for various research
topics)
COI
Conflict of Interest
COR
Contracting Officer’s Representative
COTR
Contracting Officer Technical Representative
CPRS
Computerized Patient Record System
CRADA
Cooperative Research and Development Agreement
CRADO
Chief Research and Development Officer
CSP
Cooperative Studies Program
CSR&D
Clinical Science Research and Development
CVMO
Central Office Veterinary Medical Officer
DMAP
Data Management and Access Plan
DoD
Department of Defense
DSMB
Data and Safety Monitoring Board
DUA
Data Use Agreement

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 22 of 149
EPA
Environmental Protection Agency
ePROMISe
Enterprise Project Management Information System
eRA
Electronic Research Administration – National Institutes of Health
FACA
Federal Advisory Committee Act
FDA
Food and Drug Administration
FDS
Federal Service Desk
FRAC
Field Research Advisory Committee
FWA
Federal-wide Assurance
GAO
Government Accountability Office
GenISIS
Genomic Information System for Integrative Science
HIPAA
Health Insurance Portability and Accountability Act
HR
Human Resources
HRPP
Human Subjects Protection Program
HSR&D
Health Services Research and Development
HST&C
Human Subjects Training and Credentialing
IACUC
Institutional Animal Care and Use Committee
IBC
Institutional Biosafety Committee
ICF
Informed Consent Form
IG
Inspector General
IIR
Investigator-Initiated Research
IO
Institutional Official
IPA
Intellectual Property Agreement
IPA
Intergovernmental Personnel Agreement
IRB
Institutional Review Board
ISSO
Information System Security Officer
ITA
Initial Target Allowance
ITOC
Information Technology Oversight and Compliance
LAMb
Laboratory Animal Major Equipment

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 23 of 149
LOI
Letter of Intent
MOU
Memorandum of Understanding
MVP
Million Veteran Program
NIH
National Institutes of Health
NIHMS
National Institutes of Health Manuscript Submission System
NPC
Nonprofit Corporation
NLM
National Library of Medicine
NPPO
Nonprofit Program Office
OIG
Office of Inspector General
OI&T
Office of Information and Technology
OMB
Office of Management and Budget
OPM
Office of Personnel Management
ORD
Office of Research and Development
ORO
Office of Research Oversight
OSHA
Occupational Safety and Health Administration
OSTP
Office of Science and Technology Policy
PACT
Patient Aligned Care Team
PBM
Pharmacy Benefits Management
PCS
Patient Care Services
PMC
PubMed Central – Archive of the NIH National Library of Medicine
PO
Privacy Officer
ORPP&E
Office of Research Protections, Policy, and Education
QuERI
Quality Enhancement Research Initiative
RCO
Research Compliance Officer
RCS
Records Control Schedule
RDCC
Research and Development Computing Center
REQUIP
Research Equipment Quick Use Initiative Program
RIO
Research Integrity Officer

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 24 of 149
RISP
Research Information Security Program
RR&D
Rehabilitation Research and Development
SAM
System for Award Management
SMRB
Scientific Merit Review Board
ShEEP
Shared Equipment Evaluation Program
SORN
System of Records Notice
SRS
Subcommittee on Research Safety
TDA
Transfer of Disbursing Authority
TMS
Talent Management System – (Training modules in VA Learning University)
TTP
Technology Transfer Program
USDA
United States Department of Agriculture
VA
Department of Veterans Affairs
VATAS
Veterans Affairs Time and Attendance System
VERA
Veterans Equitable Resource Allocation
VHA
Veterans Health Administration
VINCI
VA Informatics and Computing Infrastructure
VistA
Veterans Health Information Systems and Technology Architecture
VISN
Veterans Integrated Service Network
VPN
Virtual Private Network
VMU
Veterinary Medical Unit
WOC
Without Compensation

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 25 of 149
SECTION 1 – Basic Information
What is Research?
The VA defines research as: a systematic
investigation (including research development,
testing, and evaluation) designed to develop or
contribute information to the generalizable
knowledge of a scientific discipline (or other
scholarly field of study). A systematic investigation
is an activity that is planned in advance and that
uses data collection and analysis to answer a
question.
1
The VA definition of research is consistent with the Office for Human Research Protections
(OHRP). VA research is defined as “research that is conducted by VA Investigators (serving on
compensated, WOC, or IPA appointments) while on VA time. The research may be funded by VA, by
other sponsors, or be unfunded. The research must be approved by the R&D Committee before it is
considered VA research and before it can be initiated.”
2
As was taught in high school, research begins with a hypothesis – a statement that explains or makes a
generalization about a set of facts or principles, usually forming a basis for possible experiments to
confirm its viability.
All research is driven by a protocol. A protocol describes the exact process to be undertaken for the
research study. The final results of research protocols are data and the analysis of that data. In a
business sense, the “deliverable” of a research study is its data. A protocol should be written in enough
detail so that a scientist or researcher can read it, follow it, perform it, and arrive at similar data as
anyone else. This data can be analyzed to make conclusions. The “power” of the data is driven by its
statistical significance which is driven by the quantity and variability of the data. Statisticians help
researchers understand how much data to acquire for their study to have statistical significance.
Why Does the VA have a Research Program?
Research is vital to providing the best care to our nation’s Veterans and has been codified as one of the
four main missions of the VHA. Research accomplishes this mission directly through the advancement of
science and knowledge and indirectly by attracting healthcare providers who have an interest in being
part of leading- and cutting-edge medicine.
Congress has recognized the importance of VA Research by allocating a designated source of money to
be used for the support of VA research, i.e., the VA Research Appropriation.
An excerpt from the June 2011 article, “VA Research: Improving Veterans’ Lives – A Historical Look”,
published in HSR&D, Publication Briefs:
“While the evolution of federal programs for the delivery of post-service care to Veterans is well
charted, the point at which medical research became an important consideration is less defined. No
direct act of the legislative or executive branches of government dictated that Veterans’ health care
could be enhanced with a research component. The association of research and clinical care grew
mainly from the wisdom and foresight of medical practitioners themselves. Records from early meetings
1
Program Guide: VHA Operations Activities that May Constitute Research
2
VHA Directive 1200.02, Research Business Operations

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 26 of 149
of advisors and consultants charged with addressing large-scale medical needs among Veterans after
World War I reveal gathering convictions that research could and should be integrated into Veterans’
health care. Beyond the positive benefit of relating that research to the unique medical circumstances of
Veterans, the move was received as key to reinforcing an evolving system of care. Many of these
advisors felt that making the system attractive to physicians with research interests and cultivating
relationships with medical education institutions would ensure the highest quality of care to Veterans.”
3
“In 1924 and 1925, a Medical Council was established to consider issues of care for Veterans within the
Veterans’ Bureau. This council wrestled with the notion of research investigation in the context of
Veteran care and ultimately endorsed the idea unanimously and the first Research Chief was hired.
Since the beginning, the research emphasis has been on how best to help Veterans and not on pure
academic advancement of knowledge. In the beginning, studies had no specific funding, or were funded
from the investigator’s own resources. The first centrally funded research came in 1933, for a cancer
research lab at the Hines VA Hospital in Chicago.
4
“World War II put VA research in a kind of hibernation, but post WWII launched the modern era of the
VA. In 1947, affiliations with medical schools took effect. The NIH began its grants program in 1945.
Despite difficulties within hospitals about how to integrate research, by 1952 the VA had medical
research programs at 66 hospitals with 373 employees paid from money set aside for research
generating over 800 research publications. In 1953, Research became a healthcare service on its own.
From 1954 to 1959, the research budget grew almost fourfold. Research within the VA was to continue
to grow and diversify into its [current] system.”
5
Published in June 2011, this article gives t
he accounts of VA Physicians, who are involved in research,
and claim to have a higher rate of job satisfaction.
In 2020, VA Research observed 95 years of advancing science at the VA through research.
In FY22, VA ORD supported just under 2,400 research projects nationwide, ranging from preclinical
studies to health services research, to multisite clinical trials.
The types of research that ORD sponsors include:
• Investigator-initiated research (Merit Review)
• Mentored research (Career Development)
• Large-scale, multisite clinical trials (Cooperative Studies Program)
• Research Centers
• Service-directed research
6
Researchers at VAMCs are also eligible to apply for funding from other sources such as NIH, DoD, or
industry sponsors. However, the Department of Veterans Affairs cannot accept monies from other
institutions, so these grants cannot be administered, financially, by the VAMC research office. In 1988,
Congress passed legislation that empowered VAMCs to establish VA-affiliated nonprofit corporations
(NPC). The legislation enabled the establishment of private, state-incorporated NPCs that provide a
flexible funding mechanism for the administration of non-VA funded research.
6
Additional information
on NPCs can be found in Section 3: VA Non-Profit Corporation and Section 16: Non-Profit Corporations.
3
VA Research: Improving Veterans’ Lives – A Historical Look at The Establishment of the Department of Veterans
Affairs Research and Development Program. Marguerite T. Hays, MD; Pg. 3.
4
Ibid.
5
Ibid.
6
VA Research and Development Strategic Plan: 2009-2014; pg. 19.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 27 of 149
The total amount of resources dedicated to VA Research from all sources in FY21 was more than $2
billion. In FY21 research funding for the Medical and Prosthetic Research Appropriation was $795
million. It is anticipated that FY22 resources contributing to VA Research will again exceed $2 billion. For
current information you can go to https://www.va.gov/budget/products.asp.
VA and VHA Handbooks and Directives
• Guidelines, policies and regulations for VA research are encoded in a series of
Handbooks/Directives and Program Guides (Note: Handbooks are being phased out and
replaced with either a Directive or Program Guide.). Handbooks/Directives related to Research
start with the digits 120X.XX for ORD and 1058.XX for ORO.
• VA Handbooks/Directives cover the entire VA (VHA, VBA, and NCA).
o The latest versions can be found at https://www.va.gov/vhapublications/index.cfm
o Human Resources are the 50XX series and can be found at:
https://vaww.va.gov/OHRM/HRLibrary/HRLibrary.asp
o Research Program Guides can be found at:
https://www.research.va.gov/resources/policies/handbooks.cfm
PLEASE NOTE: URLs beginning with www.va are Internet sites (accessible from any internet connected
device). URLS with vaww.va are Intranet sites (only accessible from within the VA firewall).

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 28 of 149
SECTION 2 – Getting a Research Project Approved at the “X” VA
How to Determine If a Project Meets the Definition of VA Research
What is VA Research? VA research is research that
is conducted by VA investigators – serving on
compensated, without compensation (WOC), or
Intergovernmental Personnel Act (IPA)
appointments – while on VA time and/or in VA
space. The research may be funded by the VA, by
other sponsors, or be unfunded. All VA research
must receive initial approval from the local
Research and Development R&D Committee.
(VHA Directive 1200.02, dated March 10, 2017)
Who Can Be a VA Investigator?
A VA investigator is any individual who conducts research approved by the R&D Committee while acting
under a VA appointment on VA time, including full and part-time compensated employees, WOC
employees, and individuals appointed or detailed to VA under the IPA of 1970. (NOTE: Individuals
working under a contract with VA or on a fee-for-service basis cannot be given a WOC appointment to
conduct research. Contractors and those under fee-for-service can provide clinical service or other
activities in support of VA research in accordance with their contract or agreement. Trainees (e.g.,
students, residents, or fellows of any profession) may serve as participants, but not PIs within a VA
facility.
The Process to Become a VA Investigator
Some VAMCs have a formal process for designating individuals who are eligible to be PIs. This might
include an interview with the ACOS/R&D and/or a review of the investigator’s experience and training. If
indicated, the ACOS/R&D or R&D Committee may stipulate that the investigator work with a mentor
while they gain the necessary experience. In most cases, a prospective clinical investigator must first be
granted research time by his or her Service Chief and/or their Chief of Staff for appropriate labor
mapping.
Principal Investigator Orientation
Orientation by the ACOS and/or other members of the Research Administration staff on various aspects
of project submission, compliance matters, securing research space, infrastructure support for
Information Security, Human Resources, Acquisition & Materials Management, research budgeting,
among other items goes a long way to setting a new or even “old” PI in the right direction to enable
their program to run as efficiently and effectively as possible. Meeting individually with PIs has the
advantage of imparting information to the investigator either early in their research career in the VA, or
upon entering the VA, and enables the orientation to be individualized to the type of research in which
the PI may be involved. Occasionally, having group meetings with PIs or “town halls” to go over new
policy or updated procedures that have come down from ORD or ORO or new local Medical Center
procedures are helpful to impart such information to the broad base of PIs.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 29 of 149
There is a myriad of topics that can be covered, but project submission, applying for VA funding and the
nuances of the VA intramural research program are important subjects to go over. Potential sources of
funding, both VA and non-VA, potential collaborators who may be doing similar types of work at the
Medical Center can be discussed. It is also helpful to go over topics such as:
• the VA NPCs and any rules for administration of non-VA funded projects.
• intellectual property and the role of the VA and university affiliate in intellectual property for
dual appointees.
• requirements for information security in the VA, training requirements of PIs and staff who are
engaged in research.
• procedures of who or what to notify for publication of manuscripts, abstract presentation, or
media contact.
• The unique nature of VA intramural funding and need to expend funds within a fiscal year with
no more than ORD-approved allowance for carryover of funds across fiscal years,
• VA travel requirements and procedures.
• VA time and attendance policies and procedures; and
• VA research equipment custodial requirements and expectations.
In addition to these topics, a listing of who are good points of contact in research administration in
addition to the ACOS/R&D and AO who may be of assistance in project submission, safety issues,
interaction with support services is also an excellent piece of orientation material. An item that should
be discussed with PIs is that governance of the R&D Program at the local level is a partnership between
Research Administration and the R&D Committee. PIs make up all or the majority of the voting members
of the R&D Committee and are an integral part in ensuring that the research program is fully functioning
and progressing. Thus, committee service on the various VA research committees should be broached
with the PI as a part of their commitment to ensure the success of the research program and their
responsibility to other PIs at their Medical Center.
There may be different ways to document the orientation meeting. For instance, it may be required
that an Assurance document be executed outlining general PI responsibilities and more specific one for
those who perform human research versus those performing animal or basic science research.
An example can be found at https://www.research.va.gov/resources/policies/guidance/form-inv
estigator-
assurance.doc
Additionally, a memo to the new PI can be generated, outlining responsibilities of being a PI. An example
can be found at https://www.research.va.gov/resources/policies/guidance/generic-VA-paid.doc
1. To initiate a project, contact the Research Office
The Research Office can point the PI in the correct direction as to what items must be submitted
for review and to which of the research committees would need to perform the review of the
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 30 of 149
research project. Some projects may not meet the definition of research and may be considered
quality assurance / quality improvement (QA/QI) projects. Additional information on what may
be considered QA/QI can be found in Program Guide: VHA Operations Activities that May
Constitute Research – 1200.21.
2. What subcommittee approvals are needed for the proposed project?
The R&D Committee acts as a “Board of Directors” or governance committee for the Research
Service. Part of this role includes providing oversight of internal VA subcommittees as well as
providing final determination on all initial project submissions. The subcommittees the R&D
Committee oversees may include your in-house (or other VA) IACUC, IRB, SRS, and IBC. As part
of this oversight, the R&DC is charged with reviewing the committees annually to ensure they
have the needed support, membership and resources to conduct and complete committee
work. The R&DC also has final determination of all initial submissions from both the in-house (or
other VA) subcommittees listed previously, as well as all initial submissions to external
committees of record, which might include your affiliate IRB, commercial IRBs or other federal
IRBs, external IACUC, and external IBC. Project submissions to any of the subcommittees (in-
house, other VA, or external IRBs) can be initiated simultaneously, but all relevant
subcommittee approvals must be obtained before R&D Committee approval is obtained.
a. Subcommittee for Research Safety (SRS) – This subcommittee reviews and
approves protocols for work conducted in wet lab space within the research
footprint, work with hazardous agents or other safety issues that are being
conducted at the VA.
i. Duties of the SRS are covered in VHA Directive 1200.08 “Safety of
Personnel and Security of Laboratories Involved in VA Research”.
ii. For those who are uncertain about what the project requires, contact
the AO, SRS Chair, or Research Biosafety Officer, if the facility has one,
for clarification.
iii. When using Recombinant DNA, Institutional Biosafety Committee
approval prior to commencing work is required. Contact the SRS chair
for more information.
b. Institutional Animal Care and Use Committee (IACUC) – This committee must
approve all projects involving experiments with animals.
i. VHA Handbook 1200.07 “Use of Animal in Research” is the relevant
Handbook
ii. VAMCs may have their own IACUCs or use an affiliated university’s
IACUC through an MOU.
iii. All protocols must have a veterinary consultation prior to review by the
IACUC. Location and funding of animal work will dictate what animal
committee and form is used to obtain approval. The submission,
regardless of what form/committee, should include a memo with
responses to the veterinarian consultation.
iv. P
rojects that are funded by VA Research Appropriation must be
reviewed by the VA IACUC of record, regardless of where the studies
will be performed, and use the VA Animal Component of Research
Project (ACORP) forms. In rare cases, the Affiliate animal protocol form

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 31 of 149
will be accepted, but this must be discussed with the Research Office
and IACUC Chair prior to submission. Generally, an MOU between the
VA and the Affiliate in outlining reciprocal acceptable review may be
good practice to formalize the protocol review process between
institutions.
v. P
rojects funded by sources other than VA or VA NPC where procedures
are performed at the Affiliate or where funds for the study are
administered by the Affiliate may be reviewed by the Affiliate IACUC.
c. Institutional Review Board (IRB) – all studies involving human subjects, or the
use of identified human samples or data, must be reviewed by the IRB of record
identified for that project.
i. VHA Directive 1200.05 “Requirements for the Protection of Human
Subjects in Research” is the relevant Directive.
ii. The facility’s IRB(s) of Record may include the facility’s own IRB(s), the
VA Central IRB, an IRB of another VA facility, the IRB(s) of its affiliated
medical or dental school, or an IRB of another federal agency or a
commercial IRB (e.g., Western IRB, Advarra, Sterling, etc.), through an
MOU or Reliance Agreement (see ORPP&E Single IRB web page:
https://www.research.va.gov/programs/orppe/single_irb.cfm
fo
r more
information).
iii. All p
rojects must be reviewed by the PO and ISSO.
iv. The VA Central IRB reviews multisite projects. Typically, these are
funded by VA and involve several (e.g., >3) sites. The scope of the
Central IRB is expanding to include multisite industry-sponsored trials.
The Central IRB can advise whether a protocol is appropriate for
submission for review. In Central IRB submissions, one site is the PI or
Study Chair, and they coordinate the submissions from the local site
investigators. Information on the Central IRB is located under the
heading for ORPP&E, or the Program for Research Integrity
Development and Education.
https://www.research.va.gov/vacentralirb/default.cfm.
d. Exemption Subcommittee – all studies determined to be exempt from human
subjects oversight. Due to the implementation of the 2018 revised common
rule, facilities have the option of creating a new subcommittee whose sole
purpose is to review exempt studies.
i. VHA Directive 1200.05 and 1200.01 “Research and Development
Committee” are the relevant directives.
ii. If a facility does not have an Exemption Subcommittee, then an IRB
member must first determine the study is exempt before the project is
reviewed and overseen by the R&D Committee. An IRB member will
need to review the project any time an amendment/modification is
submitted to ensure the project still meets the definition of exempt,
prior to the R&D Committee reviewing the request.
iii. If a facility has an Exemption Subcommittee, the subcommittee is the
oversight committee of record and will conduct reviews of the initial
submission as well as all modifications/amendments.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 32 of 149
NO WORK CAN BE PERFORMED
ON A PROJECT UNTIL NOTIFICATION
TO INITIATE RESEARCH
HAS BEEN PROVIDED BY
THE ACOS/R&D.
3. R&D Approval
a. ALL relevant subcommittee and R&D Committee approvals must be obtained before
the ACOS can notify the investigator that a new project can commence. The R&D
Committee will notify the ACOS/R&D that a project has met all subcommittee approvals,
as well as that of the R&D Committee to release the project so that it can be initiated.
b. Protocol submission to the Research Office. New projects are submitted in the VA
Innovation and Research Review System (VAIRRS). Every new project is required to
complete the Project Cover Sheet Wizard and for those that will include human
subjects, the IRB Information Sheet Wizard. Additional required forms are determined
by the type of project. In addition to the required forms, the study team may be
required to upload copies of any of the following forms: budget, proposal (grant, science
portion), assessment of clinical impact, “Biosafety” approvals, and external IRB
approvals. Human subjects protocols would include additional forms, such as informed
consent, HIPAA authorization/revocation, as well as others based on what is involved in
the study (e.g., drugs, devices, etc.).
Two additional documents are required in VAIRRS to assist the Information System
Security Officer and Privacy Officer with their reviews. For all projects, study teams will
need to complete and upload the Enterprise Research Data Security Plan (EDRSP) for the
Information System Security Officer review. For projects that include human subjects,
study teams will complete the 10-250 and upload it along with their study documents
for Privacy Officer review. The plan is to develop these two documents into Wizards
within VAIRRS for study teams to populate and attach to their submissions, until then
the stand-alone documents must be downloaded, populated and attached to the
submissions as appropriate.
4.
Required Training to Perform Research at the VA
There are numerous training requirements for various research categories that must be satisfied
for all research personnel (including the PI, co-investigators, technicians, coordinators, etc.). The
Research Office will help determine what trainings are necessary. More specific courses of
various research training can be found in Section 9.
a. Required Trainings
i. CITI Pr
ogram: PIs and all project staff must complete CITI training requirements
for VA. The courses required depend on the type of project they will be

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 33 of 149
conducting. CITI courses include human research, species specific animal
trainings, post procedure care of rodents, biosafety training, biosecurity
training, waste anesthetic gas training, Department of Transportation shipping
training, radiation safety training, and Conflict of Interest training (required for
all PIs and staff). The CITI courses required by the VA can be coordinated with
the same courses required by the academic affiliate. This can be done from the
CITI Main Menu where there is a link to “Click here to affiliate with another
institution.” Credit for completed courses for both institutions is given for the
same unique ID number of the training module taken. Instructions are given in
the CITI Support Center under Registration and Profile Information. There is
usually someone designated at the facility to be the CITI Coordinator. If you
don’t know who that person is at your facility, contact Alice Huang in the
CVMO’s office. Most facilities try to coordinate training requirements so there
are just one set of requirements for both VA and the affiliate.
ii. VA Specific TMS trainings:
These trainings include privacy and HIPAA
awareness, information security awareness, Technology Transfer, and annual
government ethics.
iii. Before p
ersonnel engage with human subjects, they may be required to
complete a didactic human subjects training session.
1. If CPRS access is needed, this may also require additional training as
determined by the local facility.
iv. Before personnel work with animals, they must complete training that may be
conducted through the Veterinary Medical Unit Orientation, by the Veterinary
Medical Officer, other clinical veterinarian, or Veterinary Technician.
v. Biosafety or hazardous substances training may be provided by Industrial
Hygiene or other Medical Center equivalent or the Research Biosafety Officer (if
available).
vi. If required by the facility, radiation safety orientation can be scheduled by
contacting the Radiation Safety Officer.
vii. Good Clinical Practice Training is also required for clinical trials sponsored by the
Cooperative Studies program or industry.
SECTION 3 – Finance Background
Money comes in different “buckets” for research. For example,
there are administrative funds for the administration of the
research program such as Cost Center (CC) 101. There are
program (award) funds in support of the funded research projects
awarded to your facility’s investigators. There are other funding
buckets that will be described in a later section of this chapter.
The program funds described below are Congressionally
appropriated VA R&D funds, allocated by ORD:
Program
Description
820 ORD
821 BLR&D
822
RR&D
The Government
Fiscal Year begins on
October 1 and ends on
September 30.
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 34 of 149
824
HSR&D
825 Cooperative Studies Program (CSP)
826 Million Veteran Program (MVP)
829
CSR&D
VHA also receives congressional appropriations to support Medical Care, and these may support the
research enterprise in a more global sense (i.e., not for specific projects). For example, Medical Care
dollars support the salaries of VA clinicians during their protected time for conducting VA research.
Monies cannot be moved from one appropriation to another without Congressional approval.
Subcategories of Medical appropriations include: 0160 for Medical Services; 0152 for Medical Support
and Compliance; and 0162 for Medical Facilities.
Types of Money
Cost Center 101 (CC101) Funds
A portion of the VA ORD annual budget is allocated to support the administration of the research
program at each VA facility with a research program. These funds are included in the Initial Target
Allowance (ITA) for each facility through CC101 in Program 821. Research administration activities
typically include personnel for research administration, supplies, or other types of broad research
support (not relegated to any specific research project) – CC101 funds are allocated to the Research
Service only. CC101 funds are based on the amount of VA-funded research at the specific VAMC.
The formula changed for FY 23 and has several components associated with the program including types
of committees, number of projects by type, VA funding, number of VA submissions to grants. Gov, and
number of projects.
The total CC101 distributed will be limited to an amount budgeted and approved by the CRADO. The
current year CC 101 funding amount for a particular station may vary but the intents is not to be less
than 80% of the CC 101 of the previous FY. The amount of 101 funds that are provided to a station, or
withheld from a station, may be based on the ability of a station to execute its budget – that is to be
sure that prior year (PY) funds are expended or obligated without any significant outstanding balances.
Veterans Equitable Resource Allocation (VERA) Funds
VERA dollars are allocated annually to VA medical centers and hospitals to support their operations,
including medical and personnel expenditures. The amount of VERA dollars received is directly tied to
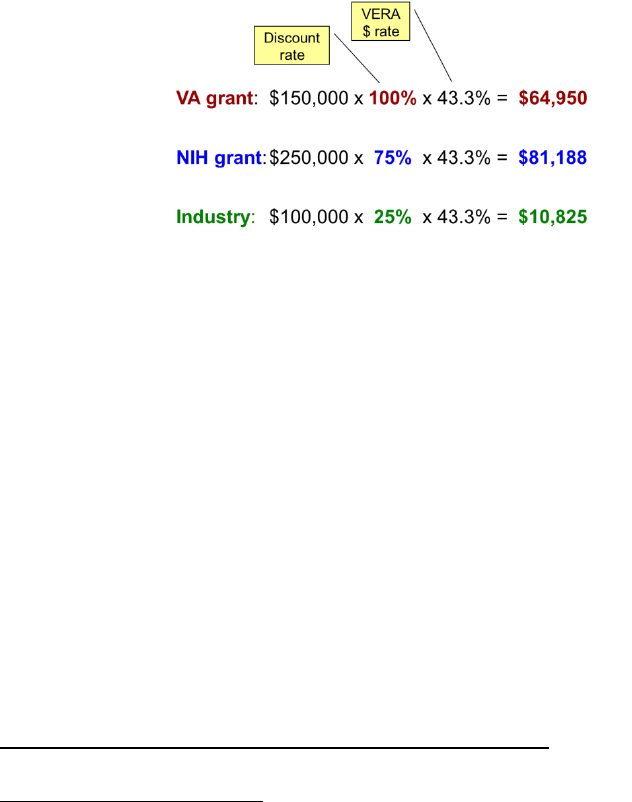
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 35 of 149
the type(s) of patient care provided by the facility, the number of Veterans it serves, the number of
unique Veteran patients seen, the complexity of patient medical problems, medical procedures
performed, and other factors. Education contributes to the VERA based on the number of trainees that a
Medical Center trains. VAMCs also receive VERA funds in proportion to the amount of research ongoing
at that site. Research support is a separate and distinct component of VERA because research programs
vary substantially across Networks and medical centers.
The total amount of Research VERA dollars available for distribution each year is fixed by VHA Finance.
To determine each VAMC’s share, the quantity of their research allocations (VA) and expenditures (non-
VA) is tabulated through the RDIS report (see below). The amount is calculated as follows:
1) Funds administered by the VAMC (which is VA funding) and funds from peer-reviewed sources
(e.g., NIH, DOD, etc.) as well as non-peer-reviewed source (e.g., pharmaceutical companies)
administered by the VA-NPCs count at 100%. Note that some types of VA Medical Care funds,
such as 870 funding, is also counted as VA research dollars (100%) in the VERA calculation.
2) Funds from peer-reviewed grant administered by the academic affiliate are discounted by 25%,
so you only count 75% of that total.
3) Funds from non-peer-reviewed sources administered by the academic affiliate (e.g., industry-
sponsored awards) are discounted by 75%, so you only count 25% of that total.
4) The adjusted or discounted amounts are tabulated for each VAMC.
5) The total amounts for all the VAMCs are summed.
6) The amount available for Research VERA is divided by the total reported expenditures in the
system to get the National Price for Research Support. For example, if the total adjusted
research expenditures across the US is $500 million and the available VERA dollars are $216.5
million, then the National Price is 43.3% - each VAMC gets 43.3 cents for every dollar of adjusted
research allocations or expenditures reported in RDIS. Please note that the VERA National
Price percent changes each fiscal year.
7) Detailed information on the VERA calculation is available at
http://vaww.arc.med.va.gov/references/faqs/faqs/faq_tt.html
8) You can see how much VERA Research support has been allocated to your site go to
http://vaww.arc.med.va.gov/. A
t the bottom of that page, you can also find archived reports
from previous years.
9) N
ote that it takes two years to transform reported research expenditures into VERA dollars, so
the amount distributed in 2022 is based on 2020 RDIS reports.
10) Note also that unfunded projects do not generate any VERA or CC101 funds, but still require the
resources of the Research Office in terms of compliance and assurance.
The Research and Development Information System (RDIS) mechanism used by VA research programs to
report annual research expenditures across the research program, including intra- and extramurally
funded projects led by VA investigators at the facility, is the source that determines the facility’s
research contribution to VERA. (See Systems for a detailed description of RDIS.) Accurate RDIS reporting
by the facility Research Office for total expenditures on each grant, award, or agreement

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 36 of 149
(pharmaceutical and biotech trials) in the Electronic Project Management and Information System
(ePROMISe) is essential to ensuring an appropriate VERA research support allocation. Allegations of
over-reporting expenditures to increase VERA research support are investigated by the Office of the
Inspector General. Under-reporting expenditures will decrease the VERA allocation for research support
at your facility. (Keep in mind: The expenditures from the previous 2 years determine the current year
VERA allocation for research support.)
The research portion of VERA can be found in the Allocation Resource Center (ARC) website
(http://vaww.arc.med.va.gov/) under the VERA tab then reports. S
elect the correct table for details.
VERA research support is disbursed at the VISN level; distribution of VERA research support at the
facility level is at the discretion of the VISN Director. While some VISN Directors keep the VERA research
support at the VISN to support research needs benefiting the entire VISN, many distribute the VERA
research support to the medical centers within the VISN based on their respective contributions to the
total research support allocation. This portion of the allocation is to be used at the Medical Center
Director’s discretion but should be used to benefit research by supporting infrastructure for the
research program. The appropriation language from Congress is that the VERA allocation is “For
necessary expenses in the administration of the medical, hospital, nursing home, domiciliary,
construction, supply and research activities...” One useful rule of thumb to remember is that the
research VERA allocation is to be used for items that benefit the research program as a whole – for
example, the IRB or HRPP program. Note that a large portion of research VERA dollars are used to cover
the salary of clinicians during the protected time that they use to conduct VA research. ORD does not
pay the salaries of clinician-investigators working on VA-funded research – this is the responsibility of
the VA Medical Center.
Award Funds
Project Awards come from the Office of Research and Development services: HSR&D, RR&D, CSR&D,
and BLR&D. These funds are directly tied to a specific award and are intended to be used by the
principal investigator (PI) designated in the proposal. If a project is delayed or if expenditures vary
substantially, the PI is required to either return the unused funds or file a project modification plan with
the funding entity and gain their official, written approval of how funds can be used alternatively or
have an extension of the project to utilize funds in the future. Information on submitting a project
modification form can be found at:
https://www.research.va.gov/resources/policies/general_admin.cfm.
Financial Systems
VistA (Veterans Health Information Systems and Technology Architecture) is an enterprise-wide
information system built around an Electronic Health Record (EHR), used through the Veterans Health
Administration (VHA). It is a collection of about 100 integrated software modules. VistA is one of the
most widely used EHRs in the world. The most significant module is the Computerized Patient Record
System (CPRS), which was released in 1997. In addition, VistA includes computerized order entry, bar
code medication administration, electronic prescribing, and clinical guidelines.
7
It also contains the
Integrated Funds Distribution, Control Point Activity, Accounting and Procurement (IFCAP/VISTA), which
contains a large portion of the financial system used throughout the VHA. Orders are normally placed in
IFCAP/VISTA and balances for each fund control point (FCP) are tracked use the FCP official menu. It is
important to have appropriate access to this system. Timecards are also processed in VATAS.
RDIS (Research & Development Information System) is a component of the ePROMISe database. It
consists of the RDIS Annual Report which contains information on all investigators that had approved
7
www.wikipedia.com / “VistA”
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 37 of 149
projects in the current reporting year (Investigator Profiles), Listing of Investigators’ VA approved
projects (Project Funding Sheet), and a compilation of Expenditures by Investigator, Project,
Administrative Agency, Cost Center, Funding Source, and Hospital Service. Previous years (up to 18
years) expenditures and summaries can be obtained from RDIS Annual Report.
What is the RDIS Annual Report?
The RDIS annual report is done following the close out of the fiscal year to report to the Research and
Development Information System (RDIS) the fiscal data that occurred at the facility for research
activities. Each station collects and submits the data for the RDIS Annual Report using ePROMISe.
ePROMISe manages data for VA-funded, non-VA funded and non-funded research projects that are
reviewed and approved by the R&DC.
One of the key components of the RDIS Annual Report is the Project Funding Sheet (PFS, previously
called “Page 20”) which is required for each R&DC-approved project (funded or non-funded, active
during any portion of the fiscal year) by Principal or Co- Principal Investigator.
A Project Funding Sheet reports the allocations/expenditures for each project in the following ways:
• For VA funded projects, report VA Research distributions (0161A1 only) made to your station
during the fiscal year.
• For projects with funds administered through and reported by the Non-Profit Corporation (NPC),
report expenditures made during the fiscal year.
• For projects with funds administered through an Academic Affiliate, report allocations (Notice of
Award) or expenditures made during the fiscal year.
• For projects with no funding, you will still need to complete a Project Funding Sheet listing $0.
There are several reports that can be generated including: Expenditure by PI noting the sources of
funding, expenditure by Administrative Agency, expenditure by Cost Center, expenditure by funding
source and expenditure by hospital service. It is due November 15 of each year, and is prepared in
collaboration with the budget analyst, PIs, the NPC, the affiliate University, and the AO.
Research Services should be updating their RDIS information throughout the year to ensure accurate
expenditure reporting of all funded awards, grants and contacts deemed VA research as they have gone
through the VA research committees process and entered in to ePROMISe before September 30th. The
Research Budget Office or Budget Personnel or AO should work with the VANPs to obtain expenditures
in support of research projects administered by the VANPs in the present VA FY. It is very important
that a mechanism be created or a point of contact at the affiliate be made (generally in the Dean’s
Office) to assist in securing information on expenditures of VA research of dual appointees when
funding is administered by the affiliate.
It is important to be vigilant about the accuracy of this report. The network (VISN) CFO and the local
facility CFO are required to certify to the report’s accuracy. You should review your projects in
ePROMISe at the end of the year to make sure everything is coded correctly. Incorrect coding will affect
your report and the medical center’s VERA allocation.
FMS (Financial Management System) is the VHA general ledger, the primary repository of VA financial
data. It categorizes spending by fiscal period, station, and account. Both labor and non-labor spending
are reported. Several reports can be obtained from Fiscal Service that assist in managing overall
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 38 of 149
balances in the control point. These include the F20 (Control Point Activity Listing) and the Status of
Allowance (SOA) reports. Many others are available upon request from the Fiscal Service. The FMS
reports can be found at vssc.med.va.gov.
The same spending may be attributed to more than one account in FMS. For example, payroll data
appear twice. One set of codes attributes labor costs to more than 70 separate categories, such as full-
time physician, registered nurse, and social worker. These can be used to calculate hourly and annual
labor costs system wide, or for specific sites. The second set divides labor costs by pay status and type,
such as regular time, overtime, benefits, and so on. There are non-labor accounts, as well. The current
list of FMS subaccount codes, also called Budget Object Codes (BOC), is available by searching VA
Handbook 4671.2 in the VA publications page (https://www.va.gov/vapubs/)
FMS data are stored at the Austin Information Technology Center.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 39 of 149
Available Resources
ORD has made a concerted effort to train the field on the many factors involved in managing research funding.
Please go to ORD Field Administrative Officer and Financial Management Resources (sharepoint.com) fo
r more
information on these resources.
FMS U
sers Guide version 5.0.1: FMS Users Guide - Financial Management System Services (va.gov) – internal to V
site.
WinRMS
The Research Management System for Windows (WinRMS) is a local SQL data based using web-based
computer software designed to assist VA Research Field Offices in administering VA research funds. It is
available to all VA Research Field Offices at no charge; many, but not all VA research programs use
WinRMS. Installation and maintenance of WinRMS should be coordinated through your local Office of
Information and Technology (OI&T). To have RMS installed contact the RDCC.
WinRMS interfaces with VistA to extract accounting information related to personnel salaries, purchase
orders (including inter-agency personnel agreements), cost transfers, and other transactions recorded
within VistA. To ensure accurate mapping between WinRMS and VistA, it is critical to use the correct
fund control point (FCP) and subaccount category when placing orders and entering other financial
transactions in VistA. WinRMS can be used to generate budget reports, perform budget projections, and
reconcile budgets. In addition to the data automatically downloaded from VistA to WinRMS, manual
entry of some data elements is required (e.g., subaccount ceilings based on ITA and TDA disbursements
and travel).
WinRMS is also an excellent resource for identifying accounting errors in research project budgets (e.g.,
when an employee is not paid correctly, purchase orders are erroneously charged to your cost
centers/FCPs). WinRMS is incredibly cumbersome to set up, but once all the data are entered, it can be
an asset for your accounting staff and can run expense reports for your investigators by grant.
RAFT – Research Analysis and Forecasting Tool
The Research Analysis Forecasting Tool, or RAFT, is a web-based software program that will help you
track all ORD allocated funds distributed to your facility. This software has replaced the monthly budget
reports that were sent manually from the Research and Development Computing Center (RDCC). The
ACOS/R&D, A/O, and/or Budget Analyst/Tech use this system to track all funds allocated throughout the
year. It is also used to provide ORD with quarterly expenditure data by project.
The Raft Budget Reports can be viewed in several different forms. You can select a Detailed Budget
Allocation or a Summary Budget Allocation. You can print quarterly allocations by Medical Center,
Project, Investigator, and by ITAs. Administrative Offices can now download their Pink Sheets, by
investigator, from this system.
RAFT enables the capability to search for a project with criteria from words contained in the abstract in
addition to Program, Account Source, Fiscal Year, and Council Meeting Date.
The RAFT expenditure reports are due on April 15
th
, July 15
th
and October 15
th
each year. Each quarter’s
cumulative FY expenditures need to be reported into RAFT at the project level. Only Current Year funds

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 40 of 149
are reported. Comments need to be entered into the report regarding spend plans for projects that are
under executing their funds. ORD will review and may request additional information.
Trainings are provided throughout the fiscal year by the Research & Development Computing Center
(RDCC).
To receive access to RAFT, please go to ORD Field Finance Resource Center - RAFT Account Request Form v2021.04
Blank (MC).pdf - All Documents (sharepoint.com) and download the RAFT access form. Follow the directions to
complete and submit.
The URL for the RAFT log in is at http://raft.research.va.gov/RAFT

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 41 of 149
Income
What is an ITA?
Every
Fiscal Year, you will receive an Initial Target Allowance (ITA). This comes in several forms including
a spreadsheet from the Fiscal Service, a downloadable report from AACS (Home (va.gov)) , or a
download from the Research Analysis and Forecasting Tool (RAFT) system. Also, you might receive a
scanned document from your local facility fiscal representative with details of the ITA. The amount of
your ITA depends on the level of VA funding allocated to the facility research program at the beginning
of each fiscal year. Throughout the year, you will receive Transfers of Disbursement Authority (TDAs)
adding or removing money from your facility. The TDAs should be tracked by the research office to
ensure that the appropriate levels of funding have been received.
What is a Control Point (aka, Fund Control Point)?
A fund control point (FCP) is where money is allocated in IFCAP/VISTA once received at the field station.
A salary FCP is a three-digit number and an All Other FCP is a 4-digit number. Each program should have
a salary FCP and an All Other FCP assigned. The Fiscal Service has the capability to create additional
FCPs, as needed. Control Points are matrixed to ACC Codes in FMS, so transactions recorded in
IFCAP/VistA are also recorded in FMS when obligated.
What is a Transfer of Disbursement Authority or TDA?
A TDA is the mechanism, or document, which sends and removes money to and from your medical
center. Normally, TDAs have a number assigned to them, and distribute money by quarters. When
money arrives on station, it is placed in the program undistributed. You will then work with fiscal to
move to the appropriate control points.
COSTING
What is a Cost Center?
Cost centers are a way of dividing expenditures within a program.
Program 821
• CC8101 – Administration
• CC8103 – Biomedical Laboratory Merit Review
• CC8105 – Veterinary Medical Unit
• CC8106 – Centrally Directed Priority Areas
• CC8108 – Career Development
• CC8109 – Other Designated Research
• CC8110 – Research Career Scientist
• CC8119 - Reimbursables
Program 822
– has CC8124 for Rehab R&D
Program 824 – has CC8134 for Health Services R&D
Program 825 – has CC8150 for Cooperative Studies Program
Program 829 – has CC8150 for Clinical Sciences R&D
Program 826 – has CC8150 for Million Veteran Program

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 42 of 149
Expense
It is important to understand that research expenditures occur in two basic types of classes:
administrative (indirect) and study-specific (direct).
For the research service to function, it also needs the support of medical care, administrative, and
facilities resources at the local level. These include housekeeping, IT equipment (PCs, network, printers,
and telephones), engineering support (HVAC, power, minor repairs, etc.), administrative staff (HR and
Fiscal), protected time for physician and other title 38 employees, etc. The debate usually centers on the
concept of what is administrative and compliance in nature versus what is in support of a specific
research study or set of studies. But at some facilities, it is difficult to get support for research
administration furniture, copy machines, and other items. If you run into this issue, seek guidance from
the network (VISN), or from other VA facilities, and bring that information to the attention of your fiscal
officer and Associate Director.
1358s and 2237s – are funding documents used to allocate funds for Research obligations.
Contracts – What are they and how are they funded?
Contracts are mechanisms to obligate money for services or equipment and are charged to the
appropriate account. Contracts involving research large equipment or other research contracts should
be submitted by the end of the Q2 of the VA fiscal year. This will allow time for review and processing by
SAO East for research equipment – or to the local and/or network business centers with respect to other
research contracts. “Late” submission (i.e., during the Q4) may not allow for adequate time to have
contracts executed.
Intergovernmental Personnel Agreements or IPAs (Covered in the Human Resources Section). The IPA
program is managed by the Federal government’s Office of Personnel Management
(https://www.opm.gov/policy-data-oversight/hiring-information/intergovernment-personnel-act/). The
program provides for the temporary assignment of personnel between the Federal Government and
state and local governments, colleges and universities, Indian (Native American) tribal governments,
federally funded research and development centers, and other eligible organizations, including Non-
Profit Research Corporations/ Foundations (NPCs) at each VA facility. IPAs allows for assignments to be
made for people who work outside the VA to be paid
with VA research funds. The
se agreements are
normally used to acquire research expertise from a
variety of sources including affiliated universities and
NPCs when the required knowledge/experience is not
readily available from normal VA recruitment channels,
especially when seeking the help/paid collaboration
from a non-citizen.
Things to remember about IPAs
1. The Employee must have been a permanent
employee of their current employer for a minimum of 90 days before being eligible for an IPA.
2. IPAs may be used for part-time, full-time, short-term, or long-term employment, but they may
not exceed 24 months at a time. Intergovernmental Personnel Act contracts can be renewed for
an additional 24 months, not to exceed 48 months. If an employee is still needed beyond 48
months, the employee must return to their employer, and remain off an IPA for 12 consecutive
months before they can participate in another IPA. VA is seeking legislative relief from this
requirement, but at present this limitation is still operative.
3. IPAs are authorized and approved by the following individuals:

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 43 of 149
• Director, CFO, manager, current employer
• Director, local VA Medical Center requiring the services
4. Each IPA may include a cover letter from the ACOS/R&D to:
• Chief, Human Resources (to ensure that all HR policies and regulations are followed
• Chief, Financial Management (to ensure that funding information listed on the contract
is accurate and that funds are available in the control point
• Chief, Logistics
• Chief of Staff
5. Funding for IPAs is through 1358s, at most facilities. However, please check with the local
VISN/fiscal office for clarification. Regardless of how the agreement will be funded, either a
1358 or 2237 should be entered in the system prior to the start of the work.
Other Contracts – Or Contracts Pertaining to Preventative Maintenance and Inspection (PMI), Service,
Equipment Leases, Animal Feed and Bedding, Animal Purchasing, etc. Each facility may have different
contract requirements. It is important to work closely with the contracting office to meet your contract
requirements as early as possible, as several issues need to be resolved before a contract can be in
place. It is important that the prospective contractor be “vendorized” – that is, they are in the System of
Awards Management (SAM) to accept federal funds. The end-user (the Research Service) must identify
what services are being sought that need to be contracted (e.g., servicing of scientific equipment, animal
feed, and bedding). Veterinarian services, for example, would require a Scope of Work that details the
services being sought, and must provide a possible source to provide those services. Do not directly
contact contractors or vendors, unless you are trying to find out if they provide the services you are
seeking. The contracting process will be managed entirely by a Contracting Office in the Contracting or
Logistics department of the facility or network (VISN). Once a contractor/vendor has been identified –
with the help of the Contracting Office – and the contract is about to begin, a 2237 must be issued prior
to the start of the contract.
It is highly recommended that the facility identifies the needs of the contract as early as possible, as it
may take several months (or longer) for a fully executed contract to be in place.
Equipment purchases – Obligations for equipment must be entered into Vista – as soon as the
equipment has been identified, and three quotes for the same item have been received from different
vendors. A 2237 for the vendor offering the least expensive item should be entered. (Please note that
the 2237 should indicate the PI’s name and the Equipment Inventory Listing or EIL assigned to that PI,
along with any relevant delivery instructions and information)
A dedicated contracting team, R&D Contracting Division, has been established to assist in research
related procurements. Information about the team along with forms and instructions can located at the
R&D Contracting Division - Customer Center
(https://dvagov.sharepoint.com/sites/vacovhacomm/admin/contracts/default.aspx)
The dedicated team can assist with research-specific contracts of $50K and above in total cost
(including all option years) and non-IT contracts. The RAC can also assist with Interagency Agreements
(IAAs) greater than $750K. The RAC is available to assist research offices during the planning stage of the
acquisition and can guide in the wiring of the contract documents that are part of the Acquisition
Package found in the above SharePoint site. There should be a local Contracting Officer’s Representative
(COR) (can be the AO or a point of contact in the Research Budget Office) to assist the Contracting
Officer with more technical points regarding specific needs and requirements of the research program
wanting to enter into a contract. Formal online training is required to be a COR. This training is
available through Federal Acquisition Institute Training Application System (FAITAS) Continuous Learning
for FAC-COR Certification via https://faitas.army.mil/Faitas/

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 44 of 149
Intragovernmental Reimbursable Agreements: Intragovernmental reimbursable agreements may be
executed within VA between different appropriations or between VA and another Federal agency.
Volume 1 Chapter 11 of VA Financial policies and Procedures (https://www.va.gov/finance/docs/VA-
FinancialPolicyVolumeIChapter11.pdf) describes in detail the utilization of these agreements.
Reimbursable agreements may be entered under various legislative authorities. They are characterized
as buy/sell monetary arrangements within or between Federal agencies and are a type of
intragovernmental transaction. VA organizations that require support will first consider support
capabilities available within their organizations, consistent with mission requirements and regulatory
authorizations before seeking other sources.
Cost Transfers: Cost transfers occur when costs are incurred in a different appropriation, program, fund
control point or station than where the cost should be accounted. To initiate a cost transfer, you must
work with your local budget office to initiate. Normally an email is sent explaining the amount to cost
transfer, where the cost was originally incurred and where the cost should be incurred. In many cases,
you are transferring costs from current year to prior year or from one program to another. It is
important to understand that you are transferring the cost from where it was incurred to where it
should be booked. For example: from current year salary to prior year salary.
Bills of Collection: Bills of Collection normally occur when the research office should be reimbursed by
the NPC or University for costs incurred. Examples include salary, animal per diems, core services etc.
To initiate a bill of collection, you must have the billing menu in VISTA. The entity being billed must be
vendorized in the VA system. Key points to remember specifically including which organization you are
billing (i.e. the university or NPC). This is important because billing the NPC goes to a different
appropriation than billing the university. You must know the form type, category, and appropriation.
Working with accounting will facilitate the generation of a bill. When billing the NPC, use the
“Additional Guidance on Implementation of the 0161x2 Fund” provided by ORD Finance.
Payroll – Are costs associated with VA personnel. Payroll costs can be found through the Personnel and
Accounting Integrated Data (PAID) system. Reports can be generated by fiscal to help balance the books.
These include PAID, gross to net, and F20.
General Post Funds
General Post Funds (GPFs) are used at the local facility. In most cases, these funds have certain
restrictions tied to the donation. Thus, a donor’s letter must be provided by the donor of the funds to
identify its use in research. Such a donor’s letter can specify which PI should be allocated those funds
and could specify the type of research that is to be undertaken. Your program will have standard
operating procedures for how to accept and expend general post funds. Sometimes GPF expenditures
are processed for approval through the R&D committee; at other facilities, the facility Director, Chief of
Staff and ACOS/R&D will provide the review and approval or disapproval.
The Principal Investigator will initiate the request to spend GPFs. Funds may be used for employee travel
provided the travel is essential to the conduct of an approved project. Funds may not be used for
salaries or fringe benefits.
GPFs are not appropriations and they do not expire, but they are restricted.
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 45 of 149
Managing Research Funding
First and foremost, and it cannot be understated – SPEND YOUR MONEY!
Too often research programs wait too late to start urging investigators to spend the money they have on
hand. To the best of your ability stay on top of your budget and continually work to reduce the amount
of last-minute spending. Spend your PRIOR YEAR money first. As you know, Research receives two-year
monies, and it is important to use up your prior year’s money first. While Research receives two-year
funds, it is important to limit the amount of carry-over into the 2
nd
year. ORD annually sets the carry-
over amount that needs to be met.
Advise ORD early of any unspent money.
If you are not going to use the money sent to you by ORD, let ORD know as soon as possible. That way
ORD can make sure that it gets spent. If the money lapses (goes unspent), it makes it very difficult to get
the same amount of money (or more!) the next year from the Congress.
1. Look in the VSSC website under the finance tab at your Status of Allowance (daily report
showing ceilings, obligation totals and balances by ACC code) and F20D (daily activity report)
reports daily (located at https://vssc.med.va.gov).
2. Develop a good relationship with your Fiscal Office and CFO.
3. Do not over-obligate your First Quarter distribution when operating in a Continuing
Resolution. (This can become tricky when working with contracts based on a 12-month scope of
work.)
VA Nonprofit Corporation
What are VA Nonprofit Corporations (NPCs)
In 1988, Congress passed Public Law (P.L.) 100-322, now codified at sections 7361-66 of title 38, United
States Code (U.S.C.), which permitted the Secretary of the Department of Veterans Affairs (VA) to
authorize the establishment of Nonprofit Research and Education Corporations (NPC) at VA medical
centers (VAMC). This laid the foundation for creation of unique partnerships to conduct VA-approved
research. P.L. 100-322 allowed the establishment of private, state-chartered, nonprofit entities to
provide flexible funding mechanisms for the administration of funds, other than those appropriated to
VA, for the conduct of VA-approved research research-related activities, or education and training at the
host VA facility.
VAMCs throughout the country have long recognized the benefit of establishing NPCs to help support
the conduct of VA-approved research and education activities. During 2020, there were 80 NPCs located
in 43 states, Puerto Rico, and the District of Columbia.
NPCs continue to obtain funding from diverse sources, including private sector companies, charitable
foundations, private individuals, state and local governments, universities, and Federal entities such as
the National Institutes of Health (NIH), Department of Defense (DoD), and Centers for Disease Control
and Prevention (CDC). Research grants administered by the NPC must be approved by the facility
Research and Development Committee and are considered VA research projects.
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 46 of 149
VA NPCs can only expend funds on behalf of a VA PI when there is an active VA-approved project either
directly tied to the proposed expenditures or in support of the PI’s active research program. Revenue
taken in by the VA NPC as indirect costs recovered from grants and contracts administered by the VA
NPC could potentially be used by Research Service in support of the overall research program. Any
amounts that go back to Research Service or via the Research and Development Committee, on behalf
of Research Service, are usually allocated by the Board of Directors of the VA NPC. (See VHA Handbook
1200.17 for detailed information about VA NPCs and their relationship to the facility research program.)
In support of VA’s research programs, in September 2012, the VA Office of General Counsel established
a new team, Specialty Team Advising Research (STAR). This is a group of VA attorneys who address the
needs of the NPCs in their role supporting VA’s approved Research and Education programs. STAR
advises on many topics including review and approve various research agreements such as Cooperative
Research and Development Agreements, nondisclosure agreements, subawards, and various
partnerships. Having this team of legal specialists has greatly enhanced VA’s research programs and
NPCs growth.
NPCs also support VA in many ways beyond administering funds. Several examples are listed below:
• Renovate and upgrade VA research infrastructure.
• Provide funds, staffing, and training support to VA and affiliate universities to help cover
Institutional Review Board requirements.
• Pay for expenses related to recruitment of research investigators to the VA system.
• Fund seed grants to new investigators to aid them in establishing their VA research careers.
• Employ support staff for VA research projects.
• Cover the cost of training VA research personnel in topics such as research compliance, good
clinical practice and board governance.
• Underwrite bridge funding for VA investigators who are between research grants awards.
• Support travel and registration fees for VA investigators to attend scientific conferences.
• Procure personnel, equipment and supplies for VA animal research facilities.
• P
rovide funds for research pharmacy staff and equipment.
• Host national educational conferences for VA personnel with incidental attendance by health
professionals from surrounding communities.
An NPC’s board members (Directors), known as the Board, are responsible for governance of the NPC in
accordance with applicable Federal and state laws and regulations, the NPC bylaws, and VA policies.
An NPC may employ individuals to carry out its purposes and may determine their compensation. NPC
employees who are given a VA Without Compensation (WOC) appointment in accordance with
paragraph 8.c.(2)(a) to provide research or education services for VA must be supervised by VA
employees while providing such services to VA.
The Board must appoint an Executive Director of the NPC. Pursuant to 38 U.S.C. 7363, the Under
Secretary for Health must concur with the appointment of the Executive Director. This responsibility has
been delegated to the Director of VA medical facility where the Single or Multi-NPC is established. This
Executive Director position cannot under any circumstances be a VA or a Federal employee.
NPC employees who are directly or indirectly involved or engaged in approved VA research or education
and training activities, and who perform such duties under the supervision of VA personnel, must have a
VA WOC appointment. If performing such duties, the NPC employee must sign a VA Intellectual Property
Agreement prior to performing such work

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 47 of 149
The NPCs are a value to the VAMC, specifically to the VA Research Services, by hiring eligible employees,
ordering supplies, funding staff for the VA Research Services and many other great benefits. The
Principal Investigators are the key to the NPC success, they bring in the research and provide for the
overhead. Customer service to the Principal Investigators is paramount for both the Research Office and
the NPC.
SECTION 4 – Human Resources (HR)
Human capital is a critical element in ensuring success in research. Generally, 70 to 80 percent of the
budget of a research grant or award is allocated to procure staff to perform work on the research
project to generate, evaluate, and assemble the gathered research data, and to then formulate a
research communication for submission to scientific meetings for presentation, and to journals for
publication. The ability to recruit skilled research employees enables a research program to be
productive to, hopefully, enable the procurement of additional research support from various funding
agencies and other sources, thus sustaining the research to be able to seek answers to scientific
questions as they arise. Inability to recruit research staff inevitably leads to a non-productive research
program that will not be sustainable. Given the type of funding available (VA, other non-VA sources),
several mechanisms to employ research staff are available where the Research Office can assist the
Principal Investigator. Anyone working in a research program at the VA must have a VA appointment or
be on a research contract. The VA’s HR policies are dictated by the Office of Personnel Management
(OPM’s) policies which apply to all Federal jobs. The relevant VA Handbook for HR staffing issues is 5005.
There are authorities that address how you are paid and authorities that address how you are appointed
from an HR perspective.
Appointment Types:

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 48 of 149
• Directly paid by VA stipend or salary, so direct appointment under either Title 38 or Title
5.
• Intergovernmental Personnel Agreement (IPA).
• Without compensation appointment authority.
• Contractors are NOT under these appointment authorities, are paid under contracts,
and therefore ARE NOT COVERED BY the Federal Tort Claim Act (FTCA). Contractors
never get FTCA coverage, and always must cover their own malpractice.
Pay Plans
• General Schedule: This pay system covers the largest group of civilian white-collar
Federal employees and is identified by the pay plan code GS. Salaries & Wages
(opm.gov). While there is base pay applicable for the U.S., pay is different in
different parts of the country based on locality even for the same grade and step.
• Fe
deral Wage System: This pay system covers the largest group of civilian blue-collar
Federal employees and is identified by the pay plan code WG.
• VM, VN and AD: These pay schedules are for employees covered under the title 38
hiring authority.
Appointment Authorities – Title 38 vs Title 5
• Title 38 positions are Physicians, Dentists, Optometrists, Podiatrists, Chiropractors,
Physician Assistants, Nurses, and Extended Function Dental Auxiliaries. Title 38 Hybrid
positions include Audiologist, Speech Pathologist, LPN, Social Worker, Nursing
Assistants, etc. (Refer to VA Directive/Handbook 5005, Part III, for a full listing). There is
a special category under Title 38 that can be used to hire research staff (see below).
• The remaining VA employees are appointed under Title 5.
Tours of Duty
The usual Tour of Duty in the VA is 8 AM to 4:30 PM, but other Tours of Duty can be applied to
an individual employee depending on the situation. Tours of duty can be added to the time and
attendance system (the request process varies by station), and once added, can be applied to
any employee whose supervisor approves that tour. All tours over six hours in duration must
include a lunch break, employees are eligible for one 15-minute break for every four hours they
work. Typically, one hour lunch breaks are not authorized, but can be approved for employees
under special circumstances.
Appointment Authorities
Competitive Status
Federal Government civilian positions are generally in the competitive civil service. To obtain a
competitive service job, you must compete with other applicants in open competition.
• Veterans' Preference gives eligible veterans preference in appointment over other
applicants.
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 49 of 149
• Temporary and term appointments are not permanent, so they do not give the
employee competitive status or reinstatement eligibility. This means they don’t
have the special advantage that internal candidates have (i.e., they do not have
status, they may not apply for permanent appointments through agency internal
merit promotion procedures, which are used for filling positions from the ranks of
current and former permanent Federal employees).
• Experience gained while employed in a temporary or term position is considered
when applying later for a permanent position.
• Veterans Recruitment Appointment (VRA) – is a special authority by which agencies
may, if they wish, appoint an eligible Veteran without competition. The candidate
must meet the basic qualification requirements for the position. A VRA appointee is
initially hired for a 2-year period. The VRA authority can be used to fill positions up
through GS-11.
Permanent
(Title 5 U.S. Code)
• Permanent employees are generally initially hired under a career-conditional
appointment (Permanent – Career-Conditional Appointment). Normally, this is the first
career-type of appointment, and the appointee must complete a 1-year probationary
period and a total of 3 years of continuous creditable service to attain a career
appointment (Permanent – Career Appointment). (Note: If the appointee already has
status as a Career employee, there is no probationary period.)
• There are limited Career/Career-Conditional positions in R&D since most of the funds
are time limited. There could be Career employees in Research Administration, but
these are determined generally on a position-by-position basis at the facility level.
• Must have a set tour of duty in VATAS.
Term
(5 CFR, part 316, subpart C)
• Term appointments (Term 5 CFR, part 316, subpart C) are time limited and competitive
in nature and announced via USAJOBS.
• This appointment lasts between 1 to 4 years, depending on the project nature, and
terminates upon completion of the project. Term appointments are only made for the
expressed duration of the project.
• Term appointments are competitive in nature. Employees who have completed four
consecutive 1-year term appointments must compete for another term appointment
(again, up to four consecutive years).
• Individuals initially appointed for 90 days, or more are eligible for annual and sick leave,
as well as health and life insurance benefits.
• M
ust have a set tour of duty in VATAS.
Noncompetitive and Excepted Appointments
Temporary
(5 CFR, part 316, subpart D)
• Temporary appointments last 1 year or less, with a specific expiration date. This
temporary appointment should be used if available funds – or workload – are not
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 50 of 149
sufficient to cover employees after 1 year, or if the remaining length of the project is
less than 1 year.
There are different limits applied to clinicians (physicians, nurses, PAs, etc.)
These restrictions do not apply to the Schedule B or Title 38 Medical Support
Authority addressed in detail below.
• These appointments can be extended up to a maximum of 2 years from the date of
initial appointment in increments of 1-year or less.
• Persons initially appointed for more than 90 days are eligible for annual and sick leave;
they do not receive health or life insurance benefits.
Temporary appointments can have an intermittent tour, meaning they don’t have a set
tour in the time and attendance system. This also requires the individuals to report the
hours they work to the appropriate timekeeper for posting.
Special Needs Appointment
(5 CFR 213.3102 (i)(2) (30/30))
Facilities may make excepted temporary limited appointments of not to exceed a duration of 30
days to meet any legitimate special need that cannot be met by another appointment authority.
These appointments may be made without regard to the general eligibility requirement in
instances when a facility determines there is a critical need to fill a position on an interim basis
pending completion of competitive examining, clearances or other procedures required for a
longer appointment. Facilities may extend the service of an employee serving under this
appointment for up to 30 additional days. No more than one appointment of a given person
may be made during any period of 12 consecutive months. These appointments are non-
competitive. Persons appointed under this authority do not receive annual or sick leave, health
or life insurance benefits.
OPM provides excepted service hiring authorities to fill special jobs or to fill any job in unusual
or special circumstances under Schedules A, B, C, and D. These excepted service authorities
enable agencies to hire when it is not feasible or not practical to use traditional competitive
hiring procedures and can streamline hiring.
Medical Support Authority
(38 USC 7405(a)(1))
• Temporary appointments for full-time staff can be made for up to 3 years at a time and
can be extended indefinitely for three-year increments as long as there is sufficient
grant funding to support the position. Eligible for all benefits.
• P
art-time and intermittent appointments are for 1 day less than 1 year and cannot be
extended. These positions are also not eligible for health insurance, FERS or TSP, but
those with a part-time tour do accrue annual and sick leave. Intermittent
appointments do not accrue leave.
• Positions must be grant funded and non-administrative (as defined by the applicable
Qualification Standard on the OPM website). Eligible positions include, but are not
limited to, Biological Science Laboratory Technician, Health Technician, Health Science
Specialist, Research Pharmacologist, etc.
• You need to be able to justify to HR why this is necessary as opposed to going through
the typical 5 CFR, part 302 and VA Excepted Board procedures. If you advertise in
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 51 of 149
USAJOBs, then you cannot turn around and use the excepted authority to select the
individual – you must follow the full 5 CFR part 302 process.
Schedule B
(Schedule B Section 213.3227(a) of the Federal Register)
Schedule B can be used for excepted appointments, meaning that you can select by name an
individual with the skills that are needed for the position. This authority is only used for
positions that are part-time or intermittent at the GS-11 level or above.
The individual must have “specialized scientific, technical, or professional skills” that will be
applied to a research project.
• Appointments are for a maximum of 3 years. They can be renewed as many times
as need to complete the research and are eligible for benefits.
• These authorities are not for administratively titled positions as defined by the
Occupational Series: See Qualification Standards Section in the OPM Website.
• Hiring with an excepted appointment authority is often faster, leading people to
(erroneously) call these “expedited appointments.”
• Full-time appointments must use Title 38 Medical Support Authority, reserving
Schedule B for part-time and intermittent appointments.
• The number of Schedule B appointments in the VA is capped at 800. Use of
Schedule B appointing authority requires approval from ORD
o Send an email to Antonio Laracuente with Cc to Nazneen Mama with the
following information:
• Name:
• Proposed appt period:
• Role on project:
• Grade Level:
• Number of 1/8ths requested:
• Citizenship Status:
• Name of Project(s)
• Se
rvice ID:
• Project end date:
• HR Staffer:
• HR Email:
Schedule A Appointments
(5 CFR 213.3102(u))
These positions are to be filled by individuals with a disability, who: 1) under a temporary
appointment have demonstrated their ability to perform the duties satisfactorily; or 2) have
been certified by counselors of State Vocational Rehabilitation agencies, or the Veterans
Administration, as likely to succeed in the performance of duties.
Title 38 and Title 38 Hybrids
(38 USC 7401(1) and 38 USC 7405(3), respectively)
Research has limited authority to directly hire individuals covered by the Title 38 appointment
authority. As previously described, this authority covers Physicians, Dentists, Optometrists,
Podiatrists, Chiropractors, Physician Assistants, Nurses, and Extended Function Dental
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 52 of 149
Auxiliaries. Physicians can only be hired under Research when they are being appointed for
their Career Development Award. Nurses can be hired for CSP-funded studies, but a Nurse
needing to be hired and paid from any other VA funding source, must first receive approval from
the funding program.
Title 38 Hybrids cover occupations that provide direct patient care but are not included under the Title
38 appointing authority. This includes Social Workers, Audiologists, Psychologists, Biomedical Engineers,
etc. Research has the authority to directly appointment all individuals covered by this appointment
authority. These appointments are temporary when appointed under Research and will have an
expiration date but may be extended as needed for as long as there is sufficient funding to support the
position.
Veterans’ Appointments
Veterans with a 30% or more disability
(5 CFR 316 subparts C or D, 5 CFR 315.707)
Market your opportunities to veterans’ organizations to leverage noncompetitive appointments
leading to conversion to career or career- conditional employment of an eligible disabled
veteran who has a compensable service-connected disability of 30 percent or more. Initial
appointment must be a temporary appointment of more than 60 days or a term appointment.
An agency may convert employee to permanent status at any time during the initial temporary
or term period.
Veterans Recruitment Appointment (VRA)
(5 CFR 307)
You can hire certain veterans using noncompetitive appointments leading to conversion to
career or career-conditional employment. A veteran can be converted to a career-conditional
appointment in the competitive service after two years of satisfactory service.
Veterans Employment Opportunities Act of 1998 (VEOA)
(5 CFR 335.106, 5 CFR 315.611)
Your talent pool broadens when you use this authority. The VEOA is a special authority that
allows eligible veterans to apply and compete for positions announced under merit promotion
procedures when the agency is recruiting from outside its own workforce. For preference
eligibles or veterans with 3 years continuous active duty service.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 53 of 149
Use of Schedule B Vs. Title 38 Medical Support Authority
Schedule B
Authority
Title 38
Medical Support Authority
Can be used for excepted
appointments
Yes Yes
Required GS level
GS11 or greater Any GS level
Individuals must have
specialized technical, scientific,
or professional skills
Yes
Yes
Can be used for administrative
positions
No No
Must be linked to a specific
research project
Yes Yes
Can be used for full-time
appointments
Technically yes, but due to limited
number of slots, this authority is
reserved for part-time and
intermittent appointees
Yes
For part-time or intermittent
appointments
Yes, can appoint for up to 3 years
with multiple renewals. Includes
benefits
Yes, but can only appoint
for 1 day less than 1 year,
with no renewals and no
benefits
Total number of appointees is
capped nationwide
Yes No
Requires approval from ORD
for initial appointment
Yes No
Required ORD approval for
renewal
No No
VA Recruitment Process in Brief
There are two types of hiring processes within R&D: Non Competitive appointments and the
competitive recruitment process. A Non-Comp is an appointment (hiring) authority that OPM can
grant agencies to fill vacancies that eliminates the recruitment/competitive hiring process (e.g.,
Schedule B, Title 38, Title 38H or Medical Support Authority, as described previously).

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 54 of 149
Hiring for any position at the VA starts with the development of a Position Description (PD) or Functional
Statement (FS) for the job to be filled. PDs are classified by an HR Classifier and are based on a points
system for the various factors in the PD, but depending on the facility, FSs may not require review by the
classification unit and may only require signature by the Supervisor (and potentially the employee).
Research positions at the GS-11 rate or above may be classified based on the Research Grade Evaluation
Guide, https://www.opm.gov/policy-data-oversight/classification-qualifications/classifying-general-
schedule-positions/functional-guides/gsresch.pdf .
In cases where it is not possible to use a non-comp, it may be necessary to competitively recruit for a
position through the Delegated Examining Office (DEO). When recruiting through the DEO, various
other forms need to be completed. These include a Recruitment Request, Job Analysis Worksheet
(JAW), and Category Rating (CR) Form. The JAW (VA form 0938) lists the tasks and competencies
associated with the position based on the PD, as well as any quality ranking or selective placement
factors being requested. The CR form establishes candidate categories of Best Qualified, Highly
Qualified, Well Qualified, and Qualified for GS positions and Best Qualified, Well Qualified, and
Qualified for Wage Grade positions. This packet of information is then forwarded by the HR Specialist
to the DEO, who generates a draft vacancy announcement and assessment questionnaire for approval
by Research Service. The tasks listed in the JAW are used as the application questions posted
electronically on USA Staffing in the assessment questionnaire. Candidates deemed to be Best
Qualified, based on the candidate’s online responses, are placed on a Certification of Eligibles (“Cert”)
list. Consideration for selective placement factors (SPFs, must have skills at the time of hire that can’t
be gained on the job within the first 90-days of hire) and quality ranking factors (QRFs, these are nice to
have skills that can be gained on the job within the first 90-days of hire) should be given to obtain the
most qualified candidates.
If an Eligible candidate has Veterans Preference, only that name will appear on the Cert for all
occupations except for those occupations covered by the Professional and Scientific standard. If no
Veterans are found qualified, or none applied, all Best Qualified applicants will appear on the Cert. The
various qualification categories can be merged if no suitable non-Veteran candidates appear on the Best
Qualified Cert to view additional candidates. The same Veterans Preference rules apply for Veterans in
other qualification categories in descending order and only the Veterans Preference individual(s) will
appear on the merged Cert. Upon receipt of the Cert, the PI or AO will then interview and select the
most qualified candidate and notify HR of the selection. At that time, a designated HR representative
will make the formal offer to the selected candidate. Neither the AO for R&D, nor the PI has the
authority to make the job offer to the candidate.
There is a site on ORD’s web page where you can advertise for positions in VA research - Employment
(va.gov).
Without Compensation Employees
Without Compensations (WOCs) are VA employees who do not receive any compensation from the VA.
In many cases, they are employed at an affiliated University or VA non-profit corporation, or they may
receive compensation from other independent funding. A WOC appointment enables these employees
to legally work at the VA facility, while complying with all R&D regulations and requirements for
researchers. WOC employees are subject to VA’s ethics and conflict of interest requirements. In cases of
emergencies such as injury or sickness related to their work, a WOC appointment entitles them to
emergency medical care at the VA. It also provides them with coverage under the Federal Tort Claim
Act.
Your HR department may be concerned about giving WOC appointments to individuals who are not US
citizens and may ask that you verify that there is no US citizen available to fill the position. This is

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 55 of 149
typically accomplished by demonstrating that you have advertised on a national level and there were no
other suitable applicants. There is a site on ORD’s web page where you can advertise for WOC positions
in VA research (See “WOC Position Announcements” under “About Us” tab.
https://www.research.va.gov/about/WOCemployment.cfm). The site clearly indicates that these are
unpaid positions with no plan for future employment at the VA.
Depending on the Medical Center and Research Service of that Medical Center, a WOC packet may have
various required elements. Items that may be included in the WOC packet completed by the
prospective WOC employee include a curriculum vitae or resume, OF-306, I-9, SF-61, evidence of
completion of various required research training courses, a WOC Intellectual Property Agreement,
evidence of enrollment in the facility Occupational Health and Safety Program, proof of tuberculosis
status confirmed by either written statement from the WOC’s employment base (University, VA non-
profits, or other), or by receiving testing from Employee Health of the VA facility, a Scope of Practice,
evidence of current visa or work permit if a non-citizen, among others. Completed packets are
forwarded to facility Human Resources for review and approval by the Chief, Human Resources, or the
designee. A WOC employee cannot legally begin work at the VA prior to receiving approval from Human
Resources.
Intergovernmental Agency Personnel Agreements
The Intergovernmental Personnel Act (IPA) enables assignments to be made for people who work
outside the VA to be paid with VA research funds. For example, a person (not the PI) with a university
appointment might be approved and funded to perform VA research, but they do not want to give up
their university appointment. An IPA is NOT a funding mechanism for personnel. This is a common
misconception. It is a mechanism for reimbursing one agency for work performed at another (a
contract). If sufficient funds for the position do not exist at the sending agency to cover the assignee,
then an IPA should not be used to pay the assignees salary. An IPA is a contract that provides for
reimbursement to the sending agency for work performed by the receiving agency by the assignee. For
this reason, the employee signs that contract, meaning that the employee is willing to be assigned under
the terms of the contract; the employing or sending agency signs that it is willing to send the employee
and continue to pay the employee at his/her normal salary rate, and the receiving agency signs
indicating that is will reimburse the sending agency at the rate set forth in the contract.
Regulatory Authorities
• The Intergovernmental Personnel Act (IPA) and its provisions are set forth in 5 CFR part 334.
The act enables the “mobility assignment” of a person with specific skills, who is working for one
eligible agency (such as, an institution of higher learning) to be “assigned” to work on a program
or project at another agency that has a need for those skills. The act was created to facilitate
benefit to both the “sending” and the “receiving” agencies, while preserving the rights and
benefits for the employee on assignment. At least one of the agencies involved in the
agreement must be a federal agency (e.g., VA).
• The program is managed by the Federal Government Office of Personnel Management
(https://www.opm.gov/policy-data-oversight/hiring-information/intergovernment-personnel-
act/
• VA has delineated its policy on IPA in VA Handbook 5005
(https://vaww.va.gov/Ohrm/HRLibrary/Dir-Policy.htm), P
art I, Section C.
Which organizations can participate in the IPA program?
VA researchers typically use IPAs to bring individuals from an outside institution into the VA. The
converse, with VA employees working at another institution, is also possible through IPAs, but is not
relevant to research and is not covered here.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 56 of 149
• An organization must be approved to participate in the IPA program.
o A list of approved organizations is available at
(https://archive.opm.gov/programs/ipa/IPA-OtherOrgList.asp
).
o In general, academic affiliates and VA-NPCs are already approved.
• Depending on the relationship between the VA and non-VA entity entering into an IPA, an
MOU may be required.
• VA can certify new organizations as IPA partners, but it is a somewhat lengthy process. Before
embarking on this process, inquire whether this organization was previously certified with
another Federal organization at any time. If so, there is no need to certify again, unless you
choose to do so. To certify a new organization, submit a request to VA OHRM with the
following items:
o Cover letter describing why the organization wishes to participation in the IPA Mobility
Program and how it’s participation would benefit both VA and the non-profit
organization requesting to participate in the program,
o Articles of Incorporation,
o Bylaws,
o Internal Revenue Non-profit Statement, and
o Any additional information describing organization’s functions related to public
management concerns of governments or universities:
o Includes professional advisory services, research, educational or developmental
services, or any other services to governments or universities concerned with public
management.
Which individuals can come to the VA on an IPA?
• The assigned candidate must have worked for the “sending” agency for at least 3 months in a
position that is considered permanent, not temporary. This designation varies from agency to
agency and should be confirmed with that agency’s Human Resources department before
requesting a mobility assignment.
o This becomes a challenge for employees of VA-NPCs since the VA cannot reimburse the
VA-NPC for the first 90 days
o This requirement is not satisfied by hiring the individual at the VA-NPC on an unpaid
basis for 90 days
• IPAs are not to be used to acquire administrative, clinical staff, or patient care services
• Students, i
ncluding graduate students, are not eligible for mobility assignment under an IPA.
Term Limits of IPAs
IPAs are meant to be temporary as a process for exchanging skills between organizations. In practice, it
benefits VA research to access the skills of individuals on IPAs for extended periods of time and
legislative relief is being sought to allow this to happen. However, at present, VA is still limited to the
terms specified by OPM
• An IPA can last up to 2 years
• An IPA can be renewed for a two-year extension
• After 4 years, the individual must return to their employer for at least 12 months before going
on another IPA
• While Federal employees may not serve more than 6 years on an IPA assignment during their
career, this limitation does not apply to private sector employees.
Setting Up an IPA
1) Verify that the ‘sending’ institution is eligible to participate in the IPA program (see above)

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 57 of 149
2) Verify that the individual in question is eligible to participate in the IPA program, e.g. that they
have worked for the “sending” agency for at least 3 months in a position that is considered
permanent.
o This ca
n be done by requesting a very simple letter on the university or NPC letter head,
signed by someone in authority (same person signing the OF-69, signatory official, head
of sponsored programs, their chief HR, etc.) that simply states, “ Mr./Ms. is an
employee of the name institution and is eligible to participate in an intergovernmental
Personnel Act (IPA) assignment.”
3) Complete an OF-
69.
o There is a detailed PowerPoint on the method for completing IPA paperwork at
https://www.research.va.gov/programs/nppo/docs/Intergovernmental-Personnel-
Act.pptx
o IPAs are authorized and approved by the following individuals:
i. Director, CFO, manager, current employer
ii. Director, local VA Medical Center requiring the services
4) Funding for IPAs is through 1358s, at most facilities. However, please check with the local
VISN/fiscal office for clarification. Regardless of how the agreement will be funded, either a
1358 or 2237 should be entered in the system prior to the start of the work.
5) VA policy does not allow the payment of overhead on IPAs (see Handbook 5005, Part I, Chapter
3, Section 2 (l))
o “l. Indirect administrative costs associated with preparing and maintaining payroll
records, developing reports, negotiating the IPA agreement, office space, furnishings,
supplies, staff support, and computer time are prohibited.”
Those on an IPA Have Federal Tort Coverage and Do Not Require a WOC Appointment
The two types of IPA assignments are appointment and detail. Under five U.S.C. § 3374(b) appointees
have FTCA coverage and under § 3374(c) detailees have FTCA coverage. The statute is slightly confusing
because § 3374 only refers to those assigned from a “State or local government.” § 3374, through §
3372, also applies to those assigned to VA from universities and NPCs. Section 3372(e)(2) (general
provisions of the IPA subpart), states:
“…an assignment of an employee of another organization or an institution of higher education to a
Federal agency, and an employee so assigned, shall be treated in the same way as an assignment of an
employee of a State or local government to a Federal agency, and an employee so assigned, is treated
under the provisions of this subchapter governing an assignment of an employee of a State or local
government to a Federal agency.”
In other words, whether from a “State or local government,” an “other organization,” or “an institution
of higher learning,” an IPA assignee to VA is subject to the provisions of § 3374, which means that all IPA
assignees from these entities have FTCA coverage. Because universities fall within the definition of “an
institution of higher learning” (see § 3372(b)(2) and 5 C.F.R. § 334.102), and NPCs fall within the
definition of “other organization” (see § 3371(4)(C)), all research assignees to VA are subject to the
provisions of § 3374, which means that they are all covered by the FTCA.
Although a WOC appointment is not REQUIRED with an IPA, there seems to be no reason that it could
not be included, if that is the local VAMC’s SOP. Many sites use the WOC intake mechanism to process
folks through background checks, key card access, and other VA systems. There is no reason to change
if your site finds this to be a useful mechanism. On the other hand, if omitting the WOC process will
expedite things, you may be able to persuade your site to modify their SOP.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 58 of 149
Joint Personnel Agreements (JPA)
Though similar to an IPA, a JPA is a contract between a VA Non-Profit corporation and a university
affiliate. At a VA where a formal Research Affiliations Agreement exists that includes the local VA non-
profit as a signatory in addition to the VA itself and the academic affiliate, JPAs can be executed to
include scientific and technical personnel but not administrative personnel. Joint Personnel Agreements
are executed on a year-to-year basis, but without time limitation, provided funding is available for the
position.
Factors to Consider When Hiring VA Employees in Research Service
• Determine the period of funding for which the position(s) will be encumbered.
• Determine if the budget for the project allows for full or part-time employees.
• Know the source of funding (where the funding for hire will be administered) for the prospective
employee – is the funding from VA appropriation, Affiliate (University), or VA Non-Profit
Corporation?
• Consider the background of the candidates when looking to hire employees – do the prospective
candidates have qualifications that meet the type of job that they will be performing?
• Consider the grade level needed to perform the work of the project.
• When recruiting or appointing a candidate with superior qualifications and/or to meet a special
need of the agency for the candidate’s service, with appropriate approvals, you may be able to set
the rate of basic pay above the minimum rate of the appropriate General Schedule (GS) grade.
• Clinical personnel need to be credentialed, meaning that they undergo a systematic process of
screening and evaluation of their qualifications and other credentials, including licensure, required
education, relevant training and experience, and current competence and health status.
o Credentialing is different from Privileging, which is the process by which licensed
independent practitioners are allowed to provide specified medical, or other patient care
services at a specific institution. In the VA, privileging is accomplished through the VetPro
online system (see Section 8
).
Request for Personnel Action
VA has implemented a program to manage positions, complete recruitment actions and initiate
SF52s (request for personnel actions). HR∙Smart and Manager Self Service (MSS) are web-based
applications supporting HR management at each facility. There are 3 primary roles:
VA Service Chief. The Service Chief role in HR∙Smart is limited only to the chief of each Service
Line/Organizational Code. The Service Chief is responsible for approving all actions for his/her
direct and indirect reports before it is routed to the HR Office.
VA Manager. The VA Manager role in HR∙Smart is designed for employees’ direct supervisors
under the service chief to initiate actions and review their employee data. This role must be
assigned to all supervisors who have at least one position reporting to him/her. If the
supervisor does not have this role the action will not flow properly.
VA Admin Officer. The Administrative Officer (AO) role in HR∙Smart was built to support the
managers in initiating actions. The current design provides access to the Personnel Office
Identification (POID) level. Due to the high level of access, the AO can only see limited data and
should only be given to trusted Administrative Officers. When selecting employee’s, the AO
should double check that they are selecting the correct employee (i.e., verify department ID).

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 59 of 149
Requests for MSS access levels should be processed through the YourHR located at the Dashboard (va.gov)
Recruitment
For direct hire requests, recruitment packages typically require the following information be
submitted in HR Smart:
• Recruitment Request
• Resume/CV or applicable application
• Position Description (PD) or Functional Statement (FS)
• Occupational Requirements (OF-178)
• Justification for use of appointment authority (MSA Justification, email from ORD for
Schedule B, etc.)
• Organizational Chart
For DEO recruitment requests, these packages typically require the following information be
submitted in HR Smart:
• Recruitment Request
• Position Description (PD)
• Occupational Requirements (OF-178)
• Job Analysis Worksheet (VA Form 0938)
• Rating Criteria Summary Form
• ORD Confirmation
• Organizational Chart
Please note that some facilities have additional processes and systems that may need to be utilized
when submitted recruitment requests, it is best to check with your assigned HR Specialist (or
strategic business unit partner) to determine what is needed for submitting a particular recruitment
request.
Disciplinary Actions and Union Interaction
There is no requirement for Union officials to be present for the performance review of union member
staff. However, if any disciplinary actions are being contemplated for an employee covered by the union,
you should contact your HR department and coordinate your efforts with them.
Union representatives should know who you are if you have union members on your staff. Developing a
collegial relationship is a good idea. Remember, your staff have the right to have a union representative
with them in disciplinary proceedings.
If an employee is not performing as required, then the employee’s supervisor needs to address the
problem. Usually, issues are minor and can be handled in an informal one on one meeting. Remember
to document everything and keep good notes, you should also follow up with the employee with a
written summary of the meeting. Sometimes issues are more problematic, and a plan needs to be

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 60 of 149
developed to address it. Work with your HR department to follow the accepted process to do this. Your
HR department will also guide you through the process if the employee needs to be separated from the
VA.
On-the-Job Injury
If you have an employee who is injured or made ill “on the job”, then – first and foremost – you must
ensure that the employee receives the help they need, as soon as possible. They may be sent to your
local employee health clinic or to the Medical Center Emergency Room. The event should be
documented by the employee, as well as by anyone else involved or witnessing the event using a Report
of Contact form. The employee is responsible for initiating an incident report in ECOMP. Link to ECOMP:
https://www.ecomp.dol.gov/.
E
mployees will be required to register and submit their own injury report
(OSHA Form 301) and if they choose, they can then complete a CA-1 or CA-2 injury claim.
Performance Appraisals
The VA uses a Position Description-based performance appraisal system. You are required to meet with
the Title 5 and Title 38 hybrid employees you supervise three times a year. The first meeting is to initiate
the appraisal for the current rating cycle, which is done in October or within 30-days of hire. The
initiation documents that employees have a copy of their performance plan and understand their duties,
which is recorded through signature on the form. The second meeting is to document the mid-year
progress review and typically occurs in April/May of each year or mid-way between a new hires
onboarding date and close-out. This meeting can also be used to determine if any changes need to be
made to the performance plan. The third meeting is for close-out and occurs in October. This meeting
provides the employee with their final rating for the year and can also be used to initiate the next year’s
performance plan.
The form that is used by the VA is Form 0750 – Performance Appraisal Program. Some facilities have
converted to an online performance management system called ePerformance. For those facilities, the
system is used to electronically sign and document meetings on the performance appraisal form. The
system also allows the supervisor and employee to upload documents and notes to be used during the
final rating throughout the rating cycle. Other facilities still use the hard copy 0750 where signatures
can either be electronic or hard copy.
There is different timing for performance reviews for nurses, please check with HR on the process for
completing reviews of nurses. Physicians assigned to Research are typically reviewed at the end of each
fiscal year, you may want to check with HR, and the medical center Service Chief responsible for the
physician’s privileges, to determine who will be responsible for the physician's proficiency review.
Management Tip I: Do not wait for these meetings to address performance successes or opportunities
for performance improvement. Make it your goal that if a meeting is required, that none of the required
meetings referenced above contains anything that will surprise the staff you supervise.
Management Tip II: When documenting an employee’s shortcoming, be as specific as possible. A
Performance Improvement Plan (PIP) can be instituted with milestones that the employee must meet to
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 61 of 149
demonstrate improvement in work performance. It is always better to attend to employee performance
early rather than later and especially if one needs to do so during the employment probationary period
just after hiring. Letting problems linger will generally make them worse and may lead to unwanted
escalated actions. Above all, be consistent in your dealings with your employees.
Management Tip III: Performance awards for VA-paid employees on the Research appropriation will
come out of the VA Award (e.g., Merit Review) that employee is assigned to. Close monitoring of the
performance awards needs to occur otherwise funds to complete the proposed scientific work may be
short. Some facilities provide performance awards for employees who receive a Fully Successful rating
or better, others only provide performance awards for Excellent or higher. You will want to check with
your local HR department to determine which rating is eligible for a performance award and the
amounts for those awards.
Incentive Awards
Some Research Services have the authority to process Incentive Awards, others may fall under the
Awards Program of the medical center. The HR department at your facility will have a person designated
as a coordinator for Incentive Awards and can be very helpful regarding how to process these. The form
used is VA Form 4659 – Incentive Awards Recommendation and Approval.
These awards are broken into six categories:
• Honor
• Special Contribution: An accomplishment achieved through an individual/group effort in the
form of a special act or service in the public interest connected with or related to official
government, which contributes to the efficacy, economy, or other improvement of Government
operations, or achieves a significant reduction in paperwork, or a contribution or
accomplishment in the public interest which is a nonrecurring contribution either within or
outside of the job responsibilities.
o It can be given as many times as earned.
• Superior Performance Award
• Special Case
• Time Off: An award granted as an incentive to reinforce acts by an individual/group of
employees deserving recognition. It is granted as time off from work that is not charged to any
type of leave or official duty time or authorized absence.
o Increments of 1 hour
o Minimum: 4 hours
o Maximum: 40 hours/80 hours in a year
o Must be used within 180 days of approval.
Under the Special Contribution Award, there is the “Gross-Up” box. This is where the dollar amount is
increased so that the NET award in the “Award Value” box is what the employee receives. However, this
is generally discouraged. Remember that in some instances the award will have tax implications for the
recipient. The service is charged the amount in the “Total Award Amount” box.
Quality Step Increase (QSI)
Check with your local HR Department to learn the best way to process a Quality Step Increase (QSI) for
an employee.
VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 62 of 149
A QSI is an increase to an employee’s rate of basic pay from one step of the grade to the next step that
is granted in recognition of excellence in performance during the last appraisal cycle. The purpose of
such increases is to recognize consistently high achievers by granting faster than normal step increases.
Only General Schedule (GS) employees are eligible to receive QSIs.
A QSI not only increases an employee’s base pay, but also increases the amount of retirement benefits;
the amount of Government Life Insurance for covered employees, and often results in a higher basic pay
adjustment upon promotion of the employee.
A QSI cannot be granted to an eligible employee who has received a QSI within the preceding 52
consecutive calendar weeks, or who is at step 10 of the pay grade. It can only be given at the end of the
rating period and is considered the performance award for that period.
Managing Office Schedules and Leave
As AO and ACOS, you need to understand and execute the best schedule for your administrative and
VMU staff.
You must establish procedures for staff to report in sick and request leave. You do not manage the
schedule for researchers reporting to a PI. You may be the administrative supervisor of research staff for
HR issues, but all scheduling and scientific oversight is the responsibility of the PI or lab manager. The
ACOS is the supervisor of clinical and non-clinician Principal Investigators in Research Service, be they
VA-paid or a WOC Principal Investigator.
The ACOS may also be the approver of Overtime and Compensatory time and may also be responsible
for approving Exceptions – these are items such as an employee not putting in the correct leave in a
timely fashion, putting in a late timesheet, etc.
Things to consider before approving leave include cross-coverage, inspection schedule, and overall
office staffing.
Sometimes staff work “too much” and need to be encouraged to take leave. Others take leave
frequently and have low balances. As AO, consider mentoring staff regarding the best form of work/life
balance.
If you have questions about schedules or leave, your HR (or Payroll) department should be able to
answer them.
Liaison with HR
As AO, you are an important liaison between the Research service and HR. It is important to develop
good working relationships with the HR chief and his/her subordinates. Every HR department functions
a little differently but often the HR department will designate one or more individuals to handle
Research recruitments and/or WOCs.
The facility’s ACOS, and you, will be the contacts for any EEO matters that arise.
Frequent and open communication is a good idea.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 63 of 149
Tips on Working with Human Resources:
1. It might be fruitful for the AO/ACOS to meet with the HR Director of the Medical Center and to
meet HR staff including those in classification, recruitment, processing and records, and labor
relations. Trying to map out a strategy with the HR Director to have a defined HR Specialist
assigned to do research actions would be helpful.
2. Position descriptions of technical positions are generally useable for any type of laboratory
setting. Similarly, study coordinator position descriptions are also generally useable for any type
of clinical research. Having templates for these types of positions may preclude the
development of new position descriptions for every new position.
3. Only a designated Human Resources employee (e.g., HR Specialist) can offer a job to
candidate(s) who appear on a Certification of Eligibles (“Cert”) or for direct hire requests. The
Research Service POC for HR matters (generally, the AO) will inform HR of selection(s) from the
Cert or will forward the resume/CV of the direct hire candidate. On HR has time to review the
selection (DEO or direct hire), HR will provide the tentative job offer.
4. For term employees paid from research appropriation tied to the VA Award, consideration must
be given to when the Award funding ends or runs out and the employee must be reassigned to
another research program or have their appointment terminated. It would be difficult to
reassign an employee in a health services research program to one in a laboratory and/or
animal-based program. Similarly, not all employees in a laboratory-based program will have the
requisite laboratory skills to move to another research program. The leave balance must also be
taken into consideration when deciding when an employee must be terminated for lack of
funds. Be sure to work with HR to see if HR will issue the termination letter or if the Research
Service will issue the termination letter after vetting by HR. Speaking to HR Labor Relations on
this matter way before the event must occur can go a long way to preventing allegations of
“wrongful termination” or an EEO complaint.
Sabbaticals - Local
VA Funded PIs planning to enter into sabbaticals during active project periods are required to submit a
request for a project modification (see: https://www.research.va.gov/resources/policies/guidance/ORD-
ProjectModification.pdf ) no less than 6 calendar months prior to initiation of the sabbatical for approval
to participate in the sabbatical during the project period. The request should include a plan for
continuing the research while on sabbatical, temporarily suspending the research, or transferring the
research to another PI for an interim period of time. Failure to notify ORD about a pending sabbatical
may result in termination of funding for the project.

VA ORD – AO/ACOS Guide
ver. 3.5 June 2023, replaces June 2022
Page 64 of 149
SECTION 5 – Applying for VA-funding
eRA and Merit Review
Funding for research can come from a variety of sources:
Federal, State/Local Government, Private Industry or Non-
Profit organizations. Federal sources of funding can be
intramural VA funding (primarily through VHA Office of
Research and Development (ORD) programs (see below),
occasionally through specialty initiatives with medical care
dollars for projects that meet the criteria of research, or
extramural funding from sources such as the National
Institutes of Health (NIH), Centers of Disease Control (CDC),
and Department of Defense (DOD). Extramural funding will
be administered by either the VA Non-Profit Corporation or
the affiliated university (Sections 15 and 16).
In 1952, the VA decided that providing funding for research
was one of its important goals.
8
VA intramural funding for
research is processed through ORD and is organized into
several funding PROGRAMS.
9
Eligible researchers within the
VA (see PI Eligibility below and VHA Handbook 1200.15) can
apply for VA ORD funding for protocols that fall within funding opportunities listed as Requests for
Applications (RFAs) and Solicitations on the VA ORD intranet website.
14
Different VA Awards are Available
• Merit Reviews are funding given to an individual (or co-PIs), and typically last 3 to 4 years. These
awards are similar to NIH R01 grants. Most Programs offer VA Merit Awards as a principal
mechanism of funding. The Merit Review program is an intramural, peer-reviewed funding
mechanism to support investigator-initiated research of disorders and diseases of importance to
the health of Veterans.
10
Go to the ORD Funding page for more information
(https://www.research.va.gov/funding/default.cfm).
• Career Development awards provide salary for early career investigators. These are similar to
NIH K01 and K08 awards (see more about the Career Development Program in the section on
Growing a Program).
• Research Career Scientist awards provide salary for outstanding non-clinician senior
investigators with a strong track record of VA research accomplishments and service and are like
NIH K05 awards.
• Center and COIN awards support a group of researchers in HSRD or RR&D who are working
around a common topic. In some rounds, only existing Centers can apply for renewal depending
on availability of funds. These are like NIH Program Project or Center awards.
• Pilot Award RFAs are periodically offered in select topic areas, and the RR&D Program uses the
SPiRE (Small Projects in Rehabilitation Research) Award mechanism.
8
VA Research: Improving Veterans’ Lives – A Historical Look at The Establishment of the Department of Veterans
Affairs Research and Development Program. Marguerite T. Hays, M.D.
9
VA R&D Intranet website: http://vaww.research.va.gov/programs/
10
Ibid.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 65 of 149
• Because VA is an intramural program, these funds are called “awards” not “grants.”
Several Research Funding Programs are Offered
The VA research programs are organized into the following groups:
• BLR&D (Biomedical Laboratory Research and Development) – Program 821 – conducts
research that explores basic biological or physiological principles in humans or animals but does
not involve intact human beings. For example, it includes research on animal models and
investigations of tissues, blood or other biologic specimens from humans.
11
• CSR&D (Clinical Science Research and Development) – Program 829 – conducts research that
focuses on intact human beings as the unit of examination. Examples include interventional and
effectiveness studies, clinical, epidemiological and technological studies.
• The VA Cooperative Studies Program (CSP) – Program 825 is the Division of VA Research and
Development that is responsible for the planning and conduct of large multicenter clinical trials
in the Department of Veterans Affairs.
12
Applications to the CSP program follow a separate set
of procedures from VA Merit/CDA/Pilot funding RFAs (VHA Handbooks 1205/1205.01).
• RR&D (Rehabilitation Research and Development) – Program 822 – conducts research to
discover knowledge and create innovations that restore Veterans who have become disabled
due to injury or disease to their greatest possible functional capacity in their families,
communities, and workplaces.
• HSR&D (Health Services Research and Development) – Program 824 – supports research to
improve the delivery of healthcare to Veterans. Among the areas studied are quality and
organization of care; patient access and outcomes; and cost-effectiveness. The division’s
Quality Enhancement Research Initiative (QuERI) is designed to translate research findings into
advancements in Veterans’ care. Within each funding program, RFAs offered can vary from cycle
to cycle, and instructions within a given RFA are updated almost every submission cycle, even
when the same RFA title and number is used, so it is important to always ensure that the latest
RFA is being used when submitting an application.
• Million Veteran Program (MVP) – Program 826 – supports large genomic/genetic and related
data science research using the MVP cohort and enterprise resources. This division works with
the ORD services and/or CSP on applications and RFAs that involve MVP resources (including
biospecimens and data). The process for applications works through MVP and may use the
service RFA mechanism (e.g., through BLRD or CSRD) as appropriate.
In addition to these ORD-funded programs, the VA ORD intranet website also lists current QuERI
Program RFAs (listed under HSR&D section). QuERI (Quality Enhancement Research Initiative) programs
are funded by medical care dollars, not ORD funds, and funding is therefore issued as single year
appropriated dollars, not 2-year appropriated dollars (like ORD funds).
A listing of the current RFAs can be found at RFAs and Program Announcements (va.gov). The process
for applying for and receiving VA funds for research is described at this webpage: Funding (va.gov).
Always check for the latest RFA instructions to determine which RFAs require a letter of intent (LOI) or
intent-to-submit (ITS) submission prior to the actual application submission. Typically, RRD requires an
LOI for every submission type (even a re-submission of a revised application), and HSR&D requires an ITS
for every submission type (including resubmissions). CSRD/BLRD have more selective situations in which
11
VA R&D Intranet website: http://vaww.research.va.gov/funding/
.
12
http://www.research.va.gov/resources/policies/default.cfm
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 66 of 149
LOIs are required (e.g., all CDAs, clinical trial Merits, epidemiology research), but typically these
programs will approve the LOI for the initial and revised submissions (be sure to review the LOI approval
for details on expiration dates or other conditions of acceptance for review). Whereas RRD and HSRD
LOI/ITS have deadlines about 4 to 6 weeks prior to application submission deadline, most BL/CSRD LOIs
require a lead time of 3 to 4 months before application submission.
In addition to the LOI/ITS, other documents may be required prior to application submission, refer to
the RFAs for specifics and current deadlines (e.g., non-clinician eligibility applications to BL/CSR&D,
requests to enroll non-Veterans in human subjects research, off-site waivers (REFER to other sections of
this Handbook). VHA Handbooks also provide more general policy, criteria, and guidance critical on CDAs
(1200.04, 1202.03, 1203.03), PI eligibility (1200.15, see also below), Offsite Waiver (1200.16), Research
Career Scientist Program (1202.04), RR&D (1203.04) and HSR&D (1204.01) Centers. However, these
Handbooks are not updated as regularly as the RFAs, so the most current requirements should always
be verified in the RFA posted for a given application cycle.
Eligibility
There are certain requirements that must be met, including:
• All applicants must meet criteria for employment as a VA employee, including United States
citizenship.
• Licensed clinicians must have a VA-paid clinical appointment of at least “5/8ths” time to
receive funding. Please contact the Chief of Staff at the local VA Medical Center to learn
about clinical opportunities.
ORD defines a clinician as a licensed practitioner with a doctoral degree (MD, DO, DDS, or clinical PhD,
etc.) who treats patients at a VA Medical Center (VAMC). In certain cases, ORD may agree to confer
eligibility for individuals with less than 5/8ths appointment. The local VAMC Research Office can
request such a waiver.
• For example, recipients of NIH K awards are required to spend 75% of their time at the
applicant institution (the affiliate). Thus, in a 40-hour work week, the K award recipient can
spend no more than 10 hours per week at the VA.
• Individuals with significant effort committed to other grants, such as NIH RO1s may not have
the 25 hours per week to devote to the VA for a 5/8ths appointment.
• Time commitment to administrative or clinical activities at the affiliate is not usually
accepted as a reason to issue a less than 5/8ths waiver.
Non-clinicians seeking funding from BLRD must first apply to ORD for a waiver of eligibility to become
eligible to apply for VA funding. Please check the BLRD RFA to ensure eligibility requirements.
• The guidance for applying for Ph.D. eligibility can be found at Non-Clinician Entry Guidance
on the ORD webpage
• This process is highly competitive, and eligibility is granted for a period of
• 3 years during which the investigator must successfully receive funding or reapply for
eligibility.
• Recipients of VA funding must become VA employees at a minimum of 5/8ths appointment.
• Only US citizens can apply for non-clinician eligibility. (Clinicians with a 5/8ths VA
appointment may apply regardless of their citizenship status.)
o CSRD, RRD and HSRD do not participate in a separate non-clinician PI eligibility
process. Rather, these programs review the LOI submissions and decide for each
given LOI whether an application can be submitted (this also applies to applications
from clinician investigators).

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 67 of 149
Calendar
o In exceptional circumstances, a time-limited waiver of this 5/8ths requirement may
be granted by a given Program Director (BLRD, CSRD, RRD, and HSRD), but these
waivers are program-specific, require detailed justification, and must be approved
prior to Merit application submission. Waivers are made on a case-by-case basis as
requested by the facility Director and endorsed by the ACOS for R&D and the facility
Chief of Staff. An investigator who is approved to submit a given application by one
Program (e.g., RRD) is not automatically approved to submit under that same waiver
to other Programs, rather individual applications for each Program are required, and
the conditions of approval (e.g., duration) may vary.
The calendar for submitting proposals can be found at:
Submission Calendar for All R&D Services (va.gov)
Submission Process
The submission process for most VA funded research is done electronically. The process begins with
ensuring that an Investigator has a Commons ID (CID) in eRA Commons with an affiliation to your
medical center. Instructions can be found here.
The researcher identifies the RFA or Solicitation that for which they wish to apply. The VA SF-424
APPLICATION GUIDE is the guide that helps the investigator to complete the elements of a successful
application. The SF-424 will give way to the NIH’s Application Submission System and Interface for
Submission Tracking (ASSIST) wh
ere the Investigator submits his/her proposal directly and the Research
Office only signs off on the submission.
General information about VA funding can be found on the Internet. However, VA’s specific RFA’s can
only be found on the Intranet and can only be accessed with VA computers.
Once the investigator has completed the application, it must be submitted through an authorized agent
in the research office. This is usually the AO, or someone delegated by the AO. Sometimes it is the
ACOS/R&D. At many facilities, there is a mechanism in place to pre-review the submission before it is
uploaded. At others, it is entirely up to the investigator to ensure that the application is complete and
correct. The eRA Commons system will scan the document for basic elements and will return a
notification of either errors or warnings. Errors indicate a “fatal flaw”, which will not let the application
proceed to submission and must be addressed. Warnings will not prevent the application from being
submitted and may or may not need to be addressed. However, there are other “fatal flaws” that are
not detected by this system, such budget caps, font size, margin size, and writing style. There are 2 days
from the time of upload to submission. This is the last window of opportunity to make any changes to
the submission.
System for Award Management (SAM)
The System for Award Management (SAM), as mentioned in Section 3 – Finance, must be active at the
Medical Center for any VA award to be submitted through the Grants.gov system. Just briefly, usually
the Supply Chain Management or Financial Management Services at the Medical Center are responsible
for setting up and maintaining SAM. However, it may be good to have someone in Research Service
review the status of SAM to ensure that the annual update is maintained for VA awards to be submitted.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 68 of 149
To determine your site’s expiration data, you can go to SAM.gov | Home, log in (you can log in using
login.gov credentials + your PIV card). You may need to set up the account when first logging in.
You can skip the “role” question, you do not need a defined role to search. After logging in, go
to the search tab (https://sam.gov/search/) and enter the DUNS number. You’ll return a list of
matching entities. The new UEI will show up under the SAM entry. If you need more details just
click on the institution name – you’ll get the full address (including ZIP+4) and congressional
district, everything you need for the performance site section of your grant applications!
Pre-Review
It is a good idea to designate an administrative research staff member to pre-review all Merit proposals
before they are submitted to ORD. Even though the upload system uses the NIH eRA Commons system,
which checks the submission for completeness, this system will not detect all errors (such as, wrong font
size, wrong margin size, and budget mistakes). Also, this system does not check the content for tone or
details addressing important elements of the RFA. Often the AO is the best person to pre-review
submissions so that it will not be rejected for an administrative or formatting error.
Another common problem with Merit proposals is because it uses the NIH’s submission system,
researchers may think that their proposal should be just like their NIH submission. It is very important to
ensure that Investigators carefully follow the RFA and VA SF-424 APPLICATION GUIDE, as they differ
from NIH RFPs.
Award
All submissions are reviewed by a panel of peers and experts in each area of research. Each submission
is given a score – The lower the score, the better. A decision is made in central office as to what score
level the funding will cover. So, in one year, a score of 13 might be funded, but in another year, funding
might only extend to scores of 11 or lower.
Once it is determined that a submission will be funded, then all the approval documentation must be
submitted. This is called the Just-in-Time (JIT) process and is completed through eRA Commons. The
research office or PI will upload the various local forms and approvals needed. However, only the
Signing Official can submit the forms for review. The Program Officer will review the documents and
either approve or require additional clarifications/changes. Funds are released to each station and the
AO and/or Budget Analyst are notified of the receipt of funds. The research can begin when the study
has R&D approval. Funds supporting the research proposal can be spent after central office sends it to
the station.
Post Award
PI’s are required to submit an annual Research Performance Progress Report (RPPR) and Final RPPR. This
is done in eRA Commons using the NIH eRA Commons RPPR Module. The annual report is due 45 days
before the next budget period start date. Notifications are sent to the PI 60 days prior to due date, 30
days prior to due date and approximately 4 days after the due date.
The Final RPPR is due within 120 calendar days of the project performance end date. The PI and Signing
Official will receive notifications 10 days after performance end date, 120 days after performance end
date and 30 days after due date.
Both the annual RPPR and Final RPPR are prepared by the PI and routed to the Research Office’s Signing
Official for submission. More information on the process can be found at:
https://www.research.va.gov/resources/RPPR.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 69 of 149
ShEEP (Shared Equipment Evaluation Program) and
LAMb (Laboratory Animal Major Equipment) Program
The purpose of the ORD Shared Equipment Program is to fund the purchase of major common resource
shared equipment, or core animal facility major equipment to be used in VAMCs to support biomedical
research on behalf of investigators associated with all ORD services.
• The ShEEP program requires in-kind partnering, or direct contribution from other sources.
• Factors considered in evaluating the requests include evidence that the equipment will be used
by multiple funded VA investigators.
• The dollar amounts available to fund these requests vary by year and additional funds often
become available late in the fiscal year. These equipment purchases involve contracts that may
take a lengthy time to process because of the dollar amounts involved ($75,000 to $600,000). It
is suggested that the research program identify their needs, complete as much of the paperwork
as possible, and have the request ready to go in anticipation of a funding announcement.
Go to http://vaww.research.va.gov/funding/rfa.cfm
a
nd search for ShEEP.
Protected Time for Research and Relationship to VERA
VHA has issued guidance on the amount of protected time recommended for various research activities.
See VHA Directive 1065. Included is a table that can be found at VA ORD Guidance for Protected Time
for Research Staff
The above table is only guidance, and each individual Medical Center may modify the suggested
amounts of protected time in accordance with its needs. This protected time, or “mitigation time”, is
blended into the overall labor mapping for clinician investigators.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 70 of 149
The Chief of Staff is responsible for staffing decisions for clinicians within the medical center. Input may
be sought form the Research service regarding the amount of VERA research dollars generated by a
given investigator’s ongoing activities. An example is provided below.
An Example for VA Merit:
Dr. Doe: 8/8 VA MD clinician faculty with:
$160,000 salary + $48,000 benefits = total cost $208,000 per year.
Dr. Doe is the Principal Investigator of a VA Merit Award with budget of $150,000 per year x 4 years
(2017-2021).
Issues to consider:
1. Dr. Doe needs protected time to conduct VA Merit-funded research. Based on the VHA Handbook
1065.01, Dr. Doe requests 3/8 protected research time – equivalent to $60,000 salary + $18,000 benefit
(total $78,000 per year).
2. As an MD faculty, Dr. Doe cannot put his/her salary on VA Merit Review (i.e., Dr. Doe’s FTEE counts
towards Medical Center FTEE with salary coming from the Medical Center).
3. VERA equivalent for $150,000 VA Merit: $150,000 x 100% x $0.55 (a sample “national price”) =
*$82,500 per year
*The Medical Center will receive $82,500 per year for direct cost expended towards this study at 2 years
after their use (e.g., 2019-2023), based on the RDIS report submitted by the Research Office at the end
of each fiscal year.
4. The clinical service is recommended to protect 3/8 FTE (15 hours a week) for Dr. Doe and find
someone who can provide coverage for this effort, or other arrangements made by the clinical service
with current available faculty.
Additional Considerations
The potential for protected research time should be discussed and planned up front when recruiting
clinician and non-clinician faculty, who wish to conduct research on VA time (whether starting with
preparation of funding application or after funding award). For faculty coming over to the VA from the
affiliate, consideration should be given to comparators between affiliate and VA annual and sick leave
differences, merit increases (as is the usual case, with the affiliate), differences in retirement programs,
as well as differences in health benefits and/or other fringe benefits.
Part-time appointments at the VA and the affiliate usually have benefits pro-rated according to the
percentage of effort at each institution. Thus, the prospective faculty member or technical research staff
(who are current affiliate employees) need to weigh in these differences prior to deciding on switching
employment, dual employment, or remaining with their current employer.
Including:
• Approval to submit funding application will need to include prior planning discussions with
Service/Section Chiefs, ACOS/R&D, COS, etc., with a plan for potential coverage if or when the
clinician faculty is funded, as well as the duration of “protected time” provided for funding
application – especially for new or early career faculty members.
• VERA funding for the Medical Center will increase with additional funded studies that are
approved by the local R&D Committee. There is not a mathematical relationship in protected
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 71 of 149
time for research in this situation, which can vary for the individual investigator and the facility.
Generally, the facility will set the maximum protected time allowed to any clinician or
investigator.
For example, if Dr. Doe has an additional NIH RO-1 funding ($250,000 per year) being conducted 50% at
the VA, but administered by the university affiliate, this will result in additional VERA financing of
$51,562.5 (based on $250,000 x 50% x 75% x $0.55), resulting in total $134,062.5 of VERA financing,
generated by Dr. Doe. Based on clinical, administrative, education, and research needs, this should
result in further discussion between the investigator, ACOS/R&D, and the Medical Center, regarding the
total protected time for research.
How to Grow a Research Program
Engaging in research and being successful in it has been a larger challenge in the current climate of
limited funding resources with more competition for funding. To be successful, one of the tenets seems
to be that successful funding for the lone investigator is declining and that collaborative research with
the contribution of multiple investigators, each lending their expertise to a specific aspect of the
proposed research, appears to now be the more accepted model. In this fashion, enlisting the
assistance of those who have cutting-edge technologies or methodologies or who can bridge the
translational research gap will greatly aid a research project. In VA Research, having a translational
model even with basic science research, or performing clinically relevant research addressing a Veteran-
centric need, are important components of a successful application for funding.
To enable the growth of a research program at the facility level, a critical mass of talented and vibrant
investigators is needed. This can be accomplished by having a balanced spectrum of: 1) well-seasoned
senior investigators who are good not only in their science but in mentoring younger investigators; 2)
mid-career investigators who will fill the role of senior investigators and are developing their skill in
mentoring and networking; and 3) junior investigators who are starting their research careers and need
the encouragement, guidance, and infrastructure to continue their excitement in science and not be
discouraged by the difficult funding climate.
Growing such a balanced research program requires the efforts and commitment not only the
ACOS/R&D, but a close partnership with Clinical Service Chiefs who can identify and recruit talented
clinician-scientists at all levels (senior, mid-career, and junior) to make a career at the VA. It is critical
that the ACOS/R&D consider meeting routinely with the Clinical Service Chief in recruitment efforts for
new clinical faculty that may want to establish a research program in the VA. The ACOS/R&D would be
able to emphasize the necessity for research benefiting Veterans as Veteran-centric type research may
be more competitive for Merit Review Award applications. In planning a new clinician recruitment, the
Clinical Service Chief would also benefit from the ACOS/R&D’s insight into the potential research
contributions and accompanying protected effort based on the types of made by the prospective
recruit. A joint effort with the Department Chair at the affiliate is also helpful to assemble the resources
needed to recruit and retain top-notch scientists at all levels within the joint VA/university environment.
Moreover, it is critical that VA facility Executive Leadership provide a supporting environment for the
conduct of research through research time mapping in DSS commensurate with the contributions to
Research VERA made by a given investigator’s funded VA-based research program. In summary, a
committed team of VA facility Research Service, VA Executive Leadership, VA Clinical leadership, and
affiliate Departmental Leadership work together to grow and maintain a healthy research program.
Attracting Experienced Scientists
Attracting senior and mid-level scientists with substantial funding and resources may be challenging,
particularly in the VA environment where the “start-up” packages typical in academia cannot be
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 72 of 149
provided, and collaboration with the affiliate is indispensable in these recruitments. For starting
investigators, the VA’s Career Development Program is an effective mechanism that would allow them
up to 6/8
th
protected time (or 8/8
th
for non-clinician) to develop an independent research program after
the conclusion of the award.
For clinician-investigators at all levels (early, mid, and senior), clinical needs of the Medical Center and
the desire to pursue a research career in the VA by the recruit could be melded using VA FTEE. In
conjunction with the Clinical Service Chief and with the approval of the Chief of Staff, a part-time VA
FTEE could be offered to the recruit. A 5/8 VA FTEE would allow for the clinician-scientist recruit to be
automatically eligible to apply for VA funding and not need to go through the 8ths waiver process. This
offer of VA clinical FTEE is often an attractive incentive that the VA can contribute to the joint
recruitment process.
For non-clinician scientists, entry into the VA’s intramural research program can be by the Career
Development route if the non-clinician scientist is no more than 5 years out of completion of the PhD
degree. For non-clinician scientists, who do not qualify for the Career Development application,
eligibility to submit for Merit review (or Pilot, CSP, or SPiRE funding) has varying requirements by
program. BLR&D has an eligibility review process for non-clinician investigators; one must first be
accepted as a non-clinician investigator before submitting any applications for VA intramural awards.
For CSR&D, HSR&D and RR&D, there is no separate eligibility process per se for the non-clinician, rather
they submit a Letter of Intent (RR&D) or Intent-to-Submit (HSR&D), and if the application is accepted for
review, then they are deemed eligible as PIs for that application.
Another critical contribution that VA facilities can often make to the recruitment process is research
space which may be more available at the VA than at the academic affiliate. The availability of space
may be an attractive means to recruit investigators (especially young investigators) to the VA and thus is
one means to growing a research program. Working with the affiliate’s Medical School Dean (perhaps
even at the level of the Associate Dean for Research) to help identify investigators in need of research
space can be helpful as well as advertising for clinician needs at the VA in conjunction with VA clinical
Service Chiefs and the Chief of Staff.
Although the larger VA research programs may have research core facilities in place to serve the VA-
based research community (and affiliate investigators as well through appropriate recharge
mechanisms), many VA facilities scientific core infrastructure support is lean. However, even at smaller
VA research facilities, affiliation with a well-established university usually offers an abundance of already
established cores, such as Imaging, Morphology, Specialized Microscopy (confocal, atomic force
microscopy, etc.), Biostatistics, Informatics, Sequencing – to name a few. For those VA investigators with
dual appointments at the affiliate, securing the ability to utilize cores at affiliate recharge rates assists
VA research programs that need specialized services not available at the VA.
To establish shared equipment and core facilities at the VA, space to house the core staff to run the
core, and for large equipment, service contracts, need to be fully considered. While the VA has the
ShEEP and LAMb equipment programs for large multi-user and VMU equipment, respectively, that
would be able to cover the cost of major equipment, finding funds to support personnel to run the core
and/or obtain a service contract (usually 10% of the cost of the equipment) may be difficult. The VA
Non-Profits (VANPs) may be of some assistance in these cases should there be sufficient funding from
overheads taken in by the VANPs. Finding sufficient coverage of these non-equipment costs from other
VA award sources may be difficult as VA Merit Review budgets in general are relatively small. Other non-
VA grants could potentially be of help in covering such costs.
Small and Medium-Sized Programs

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 73 of 149
It is also the case that some small- and medium-sized research programs may not have a university
affiliate. Growing a small program in an environment without a developed affiliate with a robust
research program of its own may be difficult, as recruitment of research-minded investigators to an
isolated VA without direct academic affiliate presents significant challenges. In such cases, it may be
more beneficial to look to other research programs within the VISN to see if a partnership can be forged
with another VISN. Such bonding could be accomplished through a sharing of research space, expertise,
or research committee work (e.g., reliance of the smaller program on the IRB, IACUC, Safety, and/or
R&D Committees of the larger program). While physical distance between Medical Centers could be a
hurdle, the use of electronic protocol submission and review processes as well as other tele- and video-
communications facilitators may help to alleviate some of the logistical problems. By using a single IRB
for both sites, this can help the smaller program grow by enabling its clinician investigators to join as a
potential additional recruitment site to a study already in place, or being submitted for funding, at the
larger facility. In this fashion, investigators with a more limited research portfolio at the smaller
program can develop their research portfolio over time through collaboration, and ultimately transition
to independence, helping the program grow its base of independent investigators.
As growing a research program implies bringing in more funded investigators, making investigators
aware of VA as well as non-VA Requests for Proposals or other funding opportunities is a critical role
that the ACOS should play. The ACOS should be familiar with the current portfolio of the Research
Service, and the strengths of the investigators so when appropriate funding opportunities or a call for
proposals comes up, those opportunities can be brought to the attention of specific investigators.
Suggesting or nominating an investigator to assist on a VA Review Board may help the investigator learn
about the VA peer review process and how the Board approaches the evaluation of an award
submission. Thus, an investigator who understands what a Board looks for in various types of
submissions (basic, translational, clinical, career development, etc.) can probably use that knowledge for
his or her own future applications, and mentor other younger VA investigators on the process. In
addition, by fully understanding the scope of the VA and affiliate research program and the investigator
roster at both, an ACOS can serve as a facilitator of collaboration, to assist investigators in establishing
the necessary interactive research proposals that are increasingly the model of choice of major funding
agencies, including NIH and VA intramural research.
Growing a Program - Investigator Transfer
Programs can also grow by “inheriting” clinician or non-clinician investigators who move from another
VA station. Reasons for a move may be personal/family or in many instances, an VA investigator may be
recruited by another academic institution and that new academic institution has a VA affiliate. The
Investigator seeking to move to a new VA and would like to take his/her VA award(s) to the new VA
should first call the ACOS/R&D at the proposed new VA to explain the reasons for the potential move
and the feasibility that the Investigator could be accommodated regarding research space, equipment
needed (if VA equipment or other non-VA purchased equipment cannot be moved from the current VA
or academic affiliate), potential VA collaborators and other needed resources such as animal facilities,
relevant patient population, etc. available at the new VA so that the current ongoing research can
successfully continue at the new site. Especially for clinician-scientists, discussion with the new VA
Clinical Service Chief and Chief of Staff should be done early in the process to see if VA FTEE are
available that would be critical to ensuring a smoother transition and to meet the VA FTEE requirements
for sustaining VA funding. Additionally, administrative personnel in the new clinical service could begin
the process of onboarding with local Human Resources. Once the groundwork has been successfully set
for transfer to the new VA and the current VA Research Office has been notified of the impending
transfer, the ORD-wide Modification Form
c
an be completed and sent in to ORD for review of the
potential transfer. Once approved for transfer by ORD, the Investigator should secure the necessary
paperwork or access to the new site’s electronic project submission and review process to obtain the

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 74 of 149
new site’s research committee and subcommittee approvals. Special consideration should be given to
human subject projects when PIs propose to transfer. The wording on informed consents and other
documents may dictate the ability to transfer data to the new site.
VA funding transfer to the new site should be handled by the fiscal officers of the respective ORD R&D
Services. However, should the Investigator have funds at the current VA NPC or university affiliate, this
could potentially be more problematic. For instance, for CRADAs involving funds administered by the
current VA NPC corporation, a new CRADA would need to be established between the new VA
NPC/Medical Center and the company partner; this may require OGC review to establish the new
CRADA. Movement of funds from the VA NPC for other investigator-initiated research would need to be
approved by the funding agency (e.g., if source of funding is from a private foundation or other non-VA
governmental entity). Some completed studies that may have “left-over” funding that the funding
organization did not want returned, are usually kept in a “various donors” type of account at the VA
NPC. This funding may not want to be released by the VA NPC and it would be at the discretion of the
Board of Directors of the VA NPC whether such “various donors” funding would want to be relinquished
to the new VA NPC. Similar funding transfer issues at the university affiliate may also arise as well as
movement of affiliate purchased equipment issues that would be at the discretion of the affiliate
whether to relinquish funding and/or equipment to the new site.
In many instances, it is up to the moving Investigator to secure the funding for the actual move.
Occasionally, the receiving university affiliate may fund the move as part of the recruitment package.
Payment by VA for relocations are generally limited to new employees to Federal service. Guidance on
the rules for relocation expense coverage can be found under VA Financial Policy, Volume XIV, Chapter
8. Once at the new site, the Investigator would need to complete the hiring process by security
clearance and new badging if necessary and being put into the VA PAID system at the new VA through
Human Resources/Financial Management. IT transfer of VA data files, e-mails, etc., would also need to
be done but the receiving Research Office and/or Clinical Service may have specific contacts in the IT
Service that can assist with this. Finally, a new EIL would need to be established through A&MM for a
transferred VA equipment.
Growing a Program – Career Development Award
While VA research has been one of the shining highlights and a best kept secret in VA, a real jewel of the
VA’s intramural research program is its Career Development Award (CDA) Program. This program is
open to clinicians and non-clinicians and is an entry point into VA research. The applicant does not have
to have prior VA eights (VA FTEE) to enter the program, but should the application be successful, would
need to assume at least a 6/8ths VA FTEE upon the initiation of the award (minimum of 5/8ths for non-
clinicians in Rehabilitation R&D for the CDA- and minimum of 4/8ths for clinicians in CDA-2 for
Rehabilitation R&D). There is a caveat with clinicians applying for the CDA program in that there is a
requirement for a 2/8ths VA clinical commitment and this 2/8ths comes through the appropriate clinical
service/COS. Thus, as mentioned above, discussion amongst the prospective applicant, clinical service
chief, COS, and ACOS/R&D is critical to be sure that the clinical commitment of FTEE is feasible. The CDA
awardee needs to have a minimum of 75% research time and essentially becomes an employee of
Research Service. Since the bulk of salary for the CDA would come from Research Appropriation and
salary payment can come from only one source, generally the CDA is costed to the research
appropriation and then 2/8 clinical time and dollars are expense transferred from Research Service to
the medical care appropriation. Salary support for the CDA is generally up to 5 years (for CDA-2) and up
to 2 years for CDA-1. For the CDA-2 level, there is a modest amount of other research funding provided
per year of the award ($65,000), in addition to the salary for the CDA. Always refer to the RFA for
specific guidance on criteria for eighths and allowed budget amounts as these items may change.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 75 of 149
The eligibility requirement pertaining to application for a CDA for a clinician is that the clinician must be
no more than 10 years out of receiving his or her clinical degree, and no more than 5 years out of
completion of their last clinical training. For a non-clinician applicant, the applicant should not be more
than 5 years out of completion of their terminal degree (e.g., PhD). Applications for CDAs for all of the
ORD Services is by Letter of Intent. The eligibility criteria apply to the CDA-2 level in all of the ORD
Services. Rehabilitation R&D has the CDA-1 level where the eligibility requirement is that the applicant
is no more than 2 years out of clinical training, or if a non-clinician, no more than 2 years out of their
terminal degree. A CDA applicant must be a United States citizen.
The primary mentor of the CDA applicant should be a funded VA Investigator. Other co-mentors as
appropriate to mentor the CDA is specific skills or other aspects of career development can be solicited
and a co-mentor could be from the university affiliate or other non-VA institution.
The ACOS/R&D is responsible for monitoring the overall progress of the CDA.
Once the CDA is completed, clinician-investigators are expected to return to their clinical service and
their salary also returns to Medical Care funding. Non-clinicians completing the CDA should be eligible to
compete for further VA funding such as a Merit Review Award where salary support would be part of
the Merit Review. On occasion, clinical FTEE may not be fully available at the end of the CDA for a
clinician. A CDA Transition Program could be developed whereby Research Service could negotiate an
FTEE or two (depending on the number of CDAs) with the COS. This FTEE could be used in partnership
with the clinical service to hopefully establish at least a 5/8 VA FTEE for the individual completing the
CDA for a specified number of years (perhaps 2 to 3 years). In this way, should more clinical FTEE
become available, the clinical service can then pick up the VA eights that had been provided by Research
Service. The eights returned to Research Service can be circulated to other CDA’s completing their
award as transition FTEE.
Career Development Enhancement Award (CDEA)
The CDEA award is for senior investigators and is an equivalent of a sabbatical. Salary support can be up
to 6 months at a 50% basis. Approval is needed from the local Medical Center, especially if more than
50% effort is requested.
Career Scientist
For non-clinician scientists who have an established, independent research program at the VA for at
least 3 years and have been funded by VA awards or other national peer-reviewed research support as a
Principal Investigator for at least 6 years, demonstrated collaborations with other VA investigators,
mentored junior VA investigators, have an excellent publication track record, and who have contributed
both to the local Research program and nationally to VA research and other scholarly activities,
application can be made for a VA Career Scientist Award. Successful applicants have 5 years of salary
support outside of a VA Merit Review Award. For those Career Scientists who have demonstrated to be
highly productive leaders and achieved both national and international stature, have had continuous VA
funding for a minimum of 6 years, application can be made for the Senior Career Scientist position.
Successful a
pplicants will have 7 years of salary support outside of a VA Merit Review award. The Career
Scientist and Senior Career Scientist awards generally carry a Title 5 GS-14 and GS15 level, respectively.
These GS levels require both local Human Resources review and Central Office review.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 76 of 149
Starting a New Program
The first step is getting the Medical Center and VISN Leadership buy-in for the program. Starting any
program consumes resources. Without those commitments, the program will never start. Communicate
often - keep leadership involved every step of the way.
You need to understand the culture at your medical center. You must have dialogue between your office
and the potential researchers in your medical center. Many times, leadership wants a research program,
but they don’t fully understand the impact that it will have on workload for providers and other key
medical center staff. There needs to be a level of commitment from these key clinical areas such as
medicine, surgery, and pharmacy. These areas are typically where the impact of research will be felt (i.e.
protected research time). Chiefs of these services need to be able to plan for clinical metrics and
distribution of workload.
Space is another area of concern within most medical centers. Are there any dedicated wet labs, animal
facilities, or will it only be a clinical research program? An inventory of both space and equipment
available needs to be assessed. Can some clinical used equipment such as imaging (MRI, PET, CAT), deep
cold freezers, and centrifuges be shared with research? How about clinical staff assisting in areas such as
phlebotomy or imaging?
The second step is telling ORD and ORO that your medical center wants to start the program. Let both
ORD and ORO know that your medical center is willing and able to initiate your program. Make sure that
you set up routine follow-up calls and emails with the POCs in both offices.
Once you have a plan and goal for your medical center, set up your Gantt chart or task list in a way that
makes the most sense. The list of tasks can seem daunting. However, setting realistic goals and setting
sensible benchmarks will help you to focus your energy.
Never be afraid to ask questions. There is always someone who has been through this and can help you
when you feel you’ve lost your way. Please rely on your colleagues for advice and assistance.
Facility Startup of
Gantt
Research Program.d chart_starting a new
This is a breakdown of tasks from the local, regional, and national levels of VA.
From a local perspective:
1. Obtain support from facility leadership/MC Director to pursue a research program.
2. Obtain local and VISN leadership approval (submit a business plan to the VISN).
3. Modify your medical center organizational structure aligning research under the Chief of Staff’s
office.
4. Identify organization structure for committee setup (SRS, IRB, R&D, IACUC).
• Obtain any waivers needed from the CRADO.
5. Identify mechanism for Research Compliance Officer oversight, training, and onboarding. Ensure
that your RCO falls under the MCD office and not the ACOS for R&D.
6. Identify the mechanism and requirements for ACOS, R&D and AO training and onboarding.
7. Ensure all parties complete FWA training.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 77 of 149
8. Update or create Medical Center manuals / policies.
9. Adopt / modify research protocol submission forms / SOPs.
10. Identify committee members; endure members complete requisite training.
11. If possible, ask an established research program if committee members/chairs can attend their
research meetings as guests.
12. Have regular meetings with academic or local stakeholders to identify potential studies for
collaboration.
13. Obtain final approval of local policies, committees, SOPs.
14. Set up accounts for SAM, Grants.gov, eRA Commons.
15. Request access to ePromise and RAFT.
From t
he VISN/National/ Affiliates:
1. Introduce the idea to VISN leadership through your facility leadership and use your business
plan as roadmap.
2. Initiate discussion with ORO and ORD to identify risks to your plan and look to them for finding
an appropriate mentor.
3. Begin discussions with academic partners. Start on policies for review between both institutions
if using an academic partner’s research committees. Make sure that meeting schedules are
shared.
4. Identify MOUs or CRADAs that will be required and draft those documents.
5. Establish routine meetings with your mentor (VA Research mentor program).
6. Involve ORO/ORD in review of local policies. These are living documents and require changes
completed in a timely manner.
7. Evaluate potential for NPC startup or partnership with established NPC.
8. Submit FWA for approval through ORD.
9. Obtain FWA approval.
10. Identify projects for collaboration with NPC or academic partner(s).
11. Decide on first studies to review
12. Request mentorship from established program in review of first studies
13. Prepare subcommittee(s) submission for first studies.
14. Review initial studies in collaboration with ORO/ORD or mentor facility.
15. Initiate first research studies
When considering starting a new program, utilize the outline below to initiate the process
new station
Outline January 202
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 78 of 149
SECTION 6 – Travel
Traveling on VA Time
One of the more confusing and more challenging events that a researcher may experience, is traveling
on “company” time. A VA traveler may encounter the “easier” scenario of traveling on VA time when
requested by VACO to undertake the Travel or the potentially more “complicated” scenario when
traveling to a scientific meeting that is not sponsored by the VA, but by external organization or Travel is
not being paid by the VA, but by another sponsor. There are multiple variables to be considered when a
VA employee travels including the purpose of the travel; whether they are on VA duty time, or annual
leave; whether the travel is domestic or international; and the source of the funding for the travel.
Are they traveling on “official VA business”? Examples:
• Attending a VA training conference
• Participating in an ORD-sponsored meeting such as a peer review panel
• Giving an official talk about VA policy using slides that have been vetted by VA administration.
o Another interpretation is that it is travel that is required as part of your VA job.
o Discussing your research at a meeting is not officially representing the VA, even if the
research is VA-funded.
o Recall that research manuscripts include a disclaimer: “This does not represent the
views of the Department of Veterans Affairs”.
o If traveling on official VA business, the traveler should be on VA time and VA is
responsible for the travel expenses. Note that a traveler might choose to pay for part of
the trip with non-VA funds, but the VA is technically “on the hook” to pay for any travel
that is required as part of a person’s VA job.
Are VA funds being used to pay for the travel?
• VA funding for travel might come from ORD. This is called “cross-funded” travel or
“alternate station funding.”
• A
nother VA station may sponsor the travel (e.g., a VA Center sponsoring a meeting
requiring VA employees from other stations to attend) and this is also deemed
“alternate station funding” or “cross-funded” travel.
Example: Participating in an ORD function such scientific panel.
• VA funding might come from the individual’s VA-funded award.
Example: Attending a scientific meeting when that travel was included in the
budget of their Merit Review award.
Example: Traveling to collect data at a remote site when that travel was
explicitly included in the proposal and in the budget for the Merit Review.
• VA funding might come from the local VAMC’s travel budget.
• In general, if VA funds are used to pay for the travel, the traveler should be on VA duty
status or VA time.
Are they traveling on VA time?
If the purpose of the trip is part of the activities for which the individual was hired by the VA, then they
may travel on VA duty status or VA time. They are not required to take annual leave.
• Example: An investigator presenting their VA research findings at a scientific meeting.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 79 of 149
• Dissemination of research findings and exchange of information at scientific meetings is an
important component of research. If the research being discussed is VA research (VA-funded, or
not-VA funded but approved by the local R&D committee as VA research), then travel could be
conducted on VA time.
• It is recognized that over the span of their career, an investigator conducts a body of research
and not all of that can be uniquely parsed into VA versus non-VA research.
General
An employee may be given authorized absence without charge to leave when:
• The activity is considered of substantial benefit to VA in accomplishing its general mission or
one of its specific functions, or
• The activity will clearly enhance an employee's ability to perform the duties of the position
presently occupied or may be expected to prospectively occupy, or
•
The basis for excusing the employee is reasonably consistent with prevailing practices of
other Federal establishments in the area concerning the same or similar activities.
•
Authorized absence is not an official VA duty status, so it is not appropriate for travel using
VA funds.
•
The amount of authorized absence that a person can take each year is limited. OPM is
currently considering limiting it to 10 days per year
Is this international or domestic travel?
Approvals for international travel while on VA duty status are relatively complex and require a
substantial amount of lead time for processing (e.g., >3 months). Some travelers choose to forego this
process and instead take annual leave. However, that is not an option if the traveler is using VA funds.
International travel on VA duty time requires the use of an “Official Passport”, which has a burgundy-
colored cover.
The VA passport office must receive the request 60 days in advance. An official VA passport can be
obtained through the VA International Travel Office ([email protected]). Add
itionally, visa
endorsement in official passport based on foreign country requirements and country clearance
information (VA Form 0900) must be submitted to the State Department.
The first time you get your burgundy Official passport, you must give them your blue passport. Thus, for
several weeks, you will have no passport at all.
If funding of the international travel is through a non-VA source, the VA 0893 form also needs to be
completed.
You can have a combination of VA time and non-VA time on a given trip.
While on VA time for foreign travel, you use the burgundy passport and while on personal time you use
the blue passport.
You must enter and leave the country on the same passport (red-red or blue-blue)
The number of days on AL must be fewer than the days on official travel
Notes from VA Foreign Travel Office

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 80 of 149
To begin the foreign travel process you must first access the Foreign Travel Portal and follow the
steps. You will be prompted to complete the VA Form 0900 Country Clearance, save (location of your
choosing) and then upload in the Foreign Travel Portal. Once submitted the international travel office
will review your VA Form 0900 and provide applicable instructions to obtain a government passport
and/or vi
sa based on your foreign travel request. This travel requires Under Secretary approval. Please
follow instructions and submit all documentation as soon as possible to prevent any unforeseen
issues.
For additional information regarding foreign travel requirements, please see VA Financial Policy,
International Travel.
If you have additional questions regarding international travel, please contact them at
Your package must be uploaded and submitted in the SharePoint no later than 60 days prior to your
departure.
• Authorized absence (AA) is not a type of leave status. If funds for this trip are being donated,
YOU M
UST request “Official Travel” NOT AA.
• In addition, if annual leave (AL) is being taken in conjunction with this request, AL CANNOT
exceed the number of official travel days being requested.
• Ti
me Zone Adjustments can only be requested IF the total travel duration is OVER 14 hours to
include layovers and the fare must be coach class.
• ALL f
unding sources must be included in the estimated cost of the trip (personal funds are a
type of funding source and if AL is being taken in conjunction, expenses from personal funds
being used while on AL must be included in the cost of the travel) and should the funds for your
travel be donated, a VA Form 0893 is required, ALL signature blocks on page 2 must be signed
and dated before your foreign travel request memo is signed by all approvers.
• On
ce you have obtained approval and signatures on your foreign travel request memo, please
submit to the Foreign Travel office via email along with all the supporting documents so that we
can review and ensure that VHA Foreign Travel Policy was correctly followed. Once we have
determined that your approval memo follows the policy, we will forward to Official Passports so
that they can release your government passport for travel.
• Is the traveler using VA funds or VA time to attend Federal non-VA-funded conference or
meeting?
Do researchers need to be on “official VA duty time” to discuss their research?
• Talking about your research is not representing the VA in an official capacity. After all, the
publications have a disclaimer that says “This does not represent the views of the
Department of Veterans Affairs”
•
You cannot talk about VA sensitive information (i.e., Veteran identifiers), which you
wouldn’t be doing anyway.
•
To talk about your VA research while not on VA time, you only need the approval of your
supervisor. That can be done as a blanket memo from the supervisor allowing you to
discuss your VA research at any time.
Are funds being donated by another entity to pay for the travel on VA time?
• Donated funds to cover travel are considered “gifts” to the VA.
•
Form 0893 is submitted to Regional Counsel describing donated funds, along with
Appendices A through D, which provide detailed information about the travel plans, funding
sources, and expenditures.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 81 of 149
•
VA Form 0893 is used to accept a gift of travel under 31 U.S.C. §1353 or 5 U.S.C. §4111 and
does NOT replace travel authorization documents.
Setting Up Official VA Travel – The Process
When the VA requests Travel for a VA employee, a Travel authorization or TWX would be sent to
the local Medical Center including the Research Office. The local Medical Center is generally
interested in what VA organization will be paying for the Travel. For Travel requested by the VA
(generally ORD or VACO), alternate station funding cost center will be provided, meaning that
the Travel funds will come from ORD or VACO. This type of Travel is generally submitted to the
VA Merit Review Board or other proposal review meetings, ORD-sponsored conferences, and
the like. Approval for the Travel should first be obtained from the traveler’s immediate
supervisor.
At some stations, the Financial Management Office needs to approve the Travel and have
funding source verified, thus some Medical Centers may have a Financial Management Office
SharePoint set up for this. Once approval(s) are obtained, the traveler needs to use the Concur
Travel System to input Travel dates, funding source, and to specify mode of transportation
(airline, train, or car); reservations can be booked through Duluth Travel in the Concur system
(https://cge.concursolutions.com/). Additionally, lodging, any local car rental (if approved), can
also be booked. The traveler should set up an account in Concur. Allowed per diem including
lodging and meals and incidentals (M&I) rates differ for varying cities and regions of the country
and can be obtained from http://www.gsa.gov/portal/content/104877.
L
odging expenses over
the per diem rate may need to be approved ahead of time and it is possible that the traveler
may be responsible for lodging rates exceeding government approved lodging rates.
Additionally, if one travels more than twice a year, a gover
nment credit card can be secured
through the Financial Management Office of the Medical Center to pay for lodging, meals, and
out of pocket expenses. Government credit cards may revert to $1 credit if not used routinely
and prior to Travel, line of credit for the Government credit card needs to be reactivated
through the Financial Management Office.
Additionally, Government credit cards cannot be used for expenses not incurred for government
Travel and must be paid in full upon receipt of the credit card statement. Once Travel is
completed, expense reports should be completed within 5 days of end of Travel. Receipts that
should be kept are airline or train ticketing receipt, lodging receipt, and local transportation
receipts. Should the traveler use his/her own vehicle to get to the airport or train station and
park his or her car at the airport or train station, mileage verification from home to station and
parking receipt is also needed for reimbursement. Please note that gratuities will only be
reimbursed up to 15%. Anything more than 15% is at the expense of the traveler.
•
It is recommended that sites DO NOT require approval of individual trips by the
R&D, Education, or other local committees when the travel is:
o
Not using any local VAMC travel funds (e.g., funded by ORD)
o
Is an itemized expense in a VA-funded Merit Review or similar award since
these expenses have already been approved by the peer review panel and
will be paid from the ORD-funded award
o
Requiring local approval in these circumstances often leads to needless
delay in the process

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 82 of 149
However, despite no local VAMC funds being used, the Medical Center may still require the
traveler to process the travel authorizations, as is required for all other types of travel.
•
Frequent (>2 trips per year) travelers should work with their Fiscal Office to be
issued a VA Travel credit card.
•
The traveler is responsible for paying all charges that are incurred to this card.
•
Training must be complete to be issued this card. This training will reinforce the
requirement that only the traveler uses the card for official VA travel, and never for
any personal use.
•
A VA travel card is used to pay for hotel, parking, meals, taxis, and other ground
transportation, while traveling on official VA business.
•
If you do not have a VA issued travel credit card, then the traveler must use their
personal credit card. When travel is complete, submit an expense report for
reimbursement.
Funding of Travel through Research appropriation may be a bit easier when working through the
local Research Office although Travel funds in general are limited and the traveler may need to
seek additional sources of funding through the academic affiliate, the VA non-profit corporation
or personal funds. Once those approvals are obtained, the traveler can put in the Travel logistics
into the Concur system.
•
Expense report (called Vouchers in CONCUR).
•
Once travel is completed, submit a travel expense report within five (5) business
days.
•
This mechanism enables the amounts charged on the VA travel credit card to be
paid directly to the issuing bank.
•
Any leftover amount can be direct deposited to a personal bank account.
•
It is important to separate all the expenditures into the proper categories, as
prompted by the system (e.g., hotel taxes are separate from room charges).
•
If you do not have a VA issued travel credit card, then the traveler must use their
personal credit card. When travel is complete, submit an expense report for
reimbursement.
•
Note Regarding Concur
Having a Point of Contact in the Research Office who has had extensive training with the Concur
Travel system is key. Most, if not all, Investigators will not be able to navigate the Concur
system except maybe to try to find appropriate air transportation times (even the official
contracted airline carrier information can sometimes be daunting). This Point of Contact can be
someone in the Budget section of Research Service, or – as may befall many Research Services –
the Research Service Administrative Officer or if there is a Center or REAP administrator, that
person could also be of valuable assistance in working with Concur. Both generating the Travel
authorizations and completing Travel vouchers can be somewhat tricky with the former being
more onerous in choosing the correct codes for type of funding source. If you cannot locate a
knowledgeable user for assistance in your Research Service office, try contacting your local
Travel Office for a recommendation. They typically know staff throughout the Medical Center,
who are heavy users and willing to assist the occasional investigator.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 83 of 149
SECTION 7 – Working with Supply Chain Management
Equipment Inventories
Supply Chain Management (SCM), formerly known as Supply or Acquisition and Materials Management
(A&MM) Service) is one of the non-clinical support services that assists Research Service in equipment
management. The Equipment Inventory Listing, or EIL (formerly, the Consolidated Memorandum
Receipt, or CMR), is kept by SCM. Equipment purchased with VA funds is listed on EILs. Equipment can
also be donated to the VA and these pieces should also be listed on an EIL. Generally, it is best to have
each individual Principal Investigator have his or her own assigned EIL. Otherwise, the ACOS/R&D will
be responsible for all VA research appropriation purchased equipment placed in individual laboratories
and/or offices.
The Research Service may have a store of equipment from Investigators who have left the VA. Such
pieces of equipment may be placed on a separate EIL under the responsibility of the ACOS/R&D.
Research Service may also have administrative equipment or non-laboratory equipment. It is good
practice to have a separate EIL for this administrative equipment. Office of Information & Technology
(OI&T) is responsible for all computers and data storage devices, printers, etc., purchased with VA IT
funding. These pieces of equipment will be on an IRM EIL and not a Research Service EIL.
Once VA-purchased equipment is received by the Medical Center’s warehouse, a Biomedical Engineering
check is done, and a Biomedical Engineering sticker is placed on the equipment. At that time, SCM
applies an inventory barcode to the equipment, generally prior to delivery of the equipment to its
designated location.
On a required annual basis, all VA-purchased equipment is accounted for by a scanning process. In brief,
VA barcodes are placed on each piece of VA purchased equipment or equipment donated to the VA.
Additionally, each room where the equipment is kept also has a barcode at the entranceway. The
entranceway barcode is first scanned, followed by scanning of each piece of VA equipment within that
room. The entranceway barcode is then rescanned upon completion of the scanning of each piece of
equipment with a VA barcode. Scanners are returned to SCM for download into their Automated
Engineering Management System/Medical Equipment Reporting System (AEMS/MERS) to track all VA
equipment.
Old, outmoded, unrepairable, or otherwise unused pieces of equipment should be turned in via a
Request, Turn-In, and Receipt for Property or Services, VA Form 2237. This form is also used to report a
misplaced and unfound, lost, or stolen piece of equipment.
When non-VA IT equipment purchased by another institution is brought onto a VA station, or put into
use on a VA station, the equipment must be first approved by the local Chief Information Officer (CIO)
and put into the AEMS/MERS system of the SCM. Generally, when such IT equipment is purchased by a
VA Non-Profit Corporation, that VA Non-Profit will donate the IT equipment to the VA so that a proper
VA equipment barcode can be obtained. However, circumstances may be different when the equipment
is purchased by the academic affiliate.
An affiliate may have its own set of rules that it could loan the equipment to the VA, rather than
donating it to the VA, or in some cases would not consider even loaning the equipment to the VA.
Should that equipment be needed by a VA investigator to be used at the VA, this will pose a problem to
the Investigator who needs the equipment in their research program. In these latter cases, a
Memorandum of Understanding between the VA and the affiliate could be generated to have affiliate
equipment situated at the VA. An alternative is to work with the local CIO to approve such affiliate
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 84 of 149
equipment to be at the VA, so that the equipment could still be tracked in AEMS/MERS, with an
understanding that once the equipment is no longer needed, it can be returned to the affiliate.
Personal equipment brought to or placed in service at the VA also needs approval, depending on the
type of equipment. Personal equipment declaration can be made on VA Form 2235. For personal IT
equipment, the CIO must be the approving official. SCM may have a separate, non-barcoded sticker to
apply to such personal equipment to identify it as being approved to be at the VA.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 85 of 149
SECTION 8 – Credentialing for Research Staff Engaged in Human Subjects
Research Projects at the XVAMC
All research staff who are licensed health care professionals permitted by the VA medical facility to
provide patient care services independently must be credentialed and privileged as defined in VHA
Handbook 1100.19. The credentialing but not privileging, requirements of VHA Handbook 1100.19 and
VA Handbook 5005 apply to those Advanced Practice Registered Nurses, Physician Assistants, and
clinical pharmacy specialists who do not practice as licensed independent practitioners, as well as
physicians, dentists, and other practitioners assigned to research or administrative positions not
involved in patient care. Only practitioners who are licensed and permitted by the VA medical facility to
practice independently may be granted clinical privileges (See VHA Handbook 1100.19, Credentialing
and Privileging). The following apply to those conducting research:
• If the local VA medical facility where the research is to be performed requires privileging to
perform a given duty (e.g., a procedure) in the clinical setting, the individual must be privileged
at that VA medical facility to perform the duty before the individual can perform that duty in the
research setting.
• If the local VA medical facility requires privileging for its staff to perform a given procedure, the
staff person performing the procedure must have privileges that would allow it. The staff
person cannot rely on the privileges of another staff member, including supervisors.
Types of Personnel Engaged in Human Subjects Research
• Principal Investigator: Responsible for all aspects of the research project.
• Personnel with direct patient/participant contact: Employees who perform procedures,
interviews, telephone calls to research subjects, or clinical interventions with patients during the
conduct of a research project.
• Personnel with indirect patient/participant contact: Employees who do not interact directly with
patients but manage and/or collect study data and PHI (i.e., retrospective chart review), or
handle previously collected human specimens for research purposes.
Responsibilities
• VA Investigators and research team members must hold a VA appointment prior to beginning
any research duties and/or contact with patients/participants.
• Research staff may only perform those activities in a research study that are allowed by the job
series to which they were appointed, have the relevant credentials and privileges, and are
allowed by their research scope of practice (or scope of work). The position description (PD) or
functional statement (FS) and performance plan should reflect the duties/activities that may be
performed in the research activity. If the PD/FS and performance plan adequately address the
duties in the specific study, a Research Scope of Practice (SCOPE) may not be necessary. If not, a
SCOPE must be completed. Note: WOCs may not have a PD or FS so would generally require a
SCOPE.
Research Scope of Practice or Scope of Work
The Research SCOPE is documentation requested from personnel engaged in human subject research,
cataloging all duties granted by the PI. The SCOPE is:
• Updated when new duties are assigned, or others deleted.
• Signed by research staff and PI.
• Assignment is appropriate, as it relates to education, experience, and training of individual.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 86 of 149
It is mandatory that research staff DO NOT perform any duties or practices beyond what is allowed in
the SCOPE, PD, or FS. For example, if your SCOPE does not list that you are permitted to obtain consent,
then you are not permitted to obtain consent. Care should be taken to ensure that research employees
without requisite clinical privileges are not practicing medicine without a license. Furthermore, health
care providers may not perform activities in a research setting that they are not credentialed to perform
in a clinical care setting.
Who Needs Clinical Privileging through VetPro?
VetPro is an Internet-enabled data bank for the credentialing of VHA health care providers that
facilitates completion of a uniform, accurate, and complete credentials file. All licensed research staff, or
those with the ability to obtain a license, must undergo the VetPro credentialing process. This pertains
to MDs, RN, NPs, PAs, LPNs, LCSWs, clinical psychologists, and other licensed personnel regardless of if
they have a VA paid, WOC or IPA appointment, who
• Must be VetPro’d before engaging in direct contact with patients.
• Must be VetPro’d if VA paid.
• Must be VetPro’d if research project has a VA offsite waiver and staff members are seeing study
subjects at that site.
and
• Foreign medical graduates who are unlicensed in the United States, or those with education
allowing for clinical practice — but are not licensed to practice in the United States — do not
need to be VetPro’d.
University collaborators who do not see patients at the VA do not need to be credentialed at the VA to
enroll subjects at the University on the same study. That collaboration must be overseen by the
University IRB and medical staff office for credentialing.
Registered Nurses
• Must be VetPro’d
• May perform office duties while waiting for VetPro if:
o The VetPro process has been initiated.
o They have immunizations record verified by XVAMC Occupational Health Services.
o They have completed all required TMS trainings.
o Are listed in the IRB protocol as study staff.
o No shadowing is permitted.
o Their Scope of Practice, or SCOPE, specifically lists only office duties that do not involve
interacting with study subjects (submissions to oversight committees such as IRB,
protocol review, or attendance of sponsor meetings). Once VetPro’d, the SCOPE should
be changed to reflect engagement in direct patient contact.
o Access to
PHI is allowed.
Human Studies Orientation
• Human Studies Orientation, or HSO, might be held at the XVAMC and could be separate from
the CITI online courses on human subjects research. This HSO should include review of PI
responsibilities, research staff responsibilities, and all XVAMC policies and procedures that
pertain to Human Subjects research.
• All research personnel engaged with human subjects research might be required to take this
course.
• All VA PIs, VA Co-Investigators, and Pharm Ds might be required to take HSO, regardless of if
they have direct or indirect human contact.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 87 of 149
• Individuals could be eligible for a waiver if they are only processing lab specimens, statisticians,
and/or they are data analysts.
How to determine if someone is engaged (or not engaged) in human subjects research
• the project takes place at XVAMC or at a site with an approved off-site waiver the project is
funded by VA
• the project is being performed in VA-leased space
• involves the individual performing research specific duties that are not part of their normal job
description (i.e., ICU nurse implementing research survey)
• they are listed in the IRB protocol as VA research staff
You are Not Engaged in human research if
• you are performing your normal clinical duties during your normal tour of duty (i.e., oncology
nurse hanging investigational chemotherapy drug, research pharmacist, phlebotomist, ECG
technician)
• y
ou are not listed in the IRB protocol. However, there are some situations where you may still
be engaged in VA research.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 88 of 149
SECTION 9 – VA Training Requirements
A designated Research Administration employee (in some stations, it is the Administrative Officer) is the
XVAMC point of contact for becoming VA authorized to conduct research. This administrator manages
the authorization process and assigns courses and forms. The courses and forms are dependent upon
what the research duties are, if there is direct or indirect contact with human subjects, if there are
biological specimens, and if the personnel are licensed. Please consult the ORD web page for specific
information on the requirement (Options for Fulfilling ORD Training Requirements (va.gov).
All Research Personnel Engaged in Human Subjects Research
• Blood-borne Pathogens and Tuberculosis – sites may use the TMS module, a local training
module, or choose to accept equivalent training from the affiliate
• Collaborative Institutional Training Initiative (CITI) Biosafety fo
r personnel that handle biological
specimens (phlebotomy/CSC lab) – annually
• C
ITI VA Human Research Modules – Initial Training
• CITI VA Human Research Modules – Refresher training required every 3 years
1. Biomed Refresher 2 – History and Ethical Principles (ID: 511) – required
2. Biomed Refresher 3 – History and Ethical Principles – Research vs. Practice
(ID: 993) – required
3. Any six from the 30 refresher modules listed in CITI
4. GCP Training (CSP and CSR&D Clinical Trials as well as some industry sponsors)
Personnel Engaged in Animal Research
• Working with the VA IACUC CITI module, every 3 years
• Species-specific CITI modules, as applicable to their work – every 3 years
• IACUC members must take the CITI Essentials for IACUC members – every 3 years
• Additional optional training models can be found under the Animal Research tab on the ORD
webpage. For IACUC members, the CVMO Office has put together a compilations of various
training scenarios that are excellent.
Personnel Engaged in Laboratory Research
• Laboratory safety training as determined by local policy. This may be either by computerized
course training or in-person training or both
• Specialized training for radioisotopes or other hazard as determined by local policy.
Training Required of all Working in VA Research
• VA Privacy and Information Security and Rules of Behavior
• VHA Pr
ivacy and HIPAA (when working with PHI or III)
• Government Ethics
• Technology Transfer Program (PIs only)
• Local sites may require additional training modules for designated employees

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 89 of 149
SECTION 10 – General Administrative Management
Some Administrative Officers have other titles (especially at larger research programs), including:
“Assistant Chief of Staff for Research and Development”, “Director of Research Operations,” or
“Business Manager”, which reflects an important characteristic of the position. Administrative Officers
may or may not have a scientific background, but their primary function is to ensure the optimal
function of the Research Service. Whereas the ACOS/R&D is the head of the Research Service, the AO is
responsible for the day-to-day operations of the program.
Additionally, many AOs have such duties as HRPP Officer or Safety Coordinator. As mentioned at the
start, there is no “one size fits all” in the world of Research AOs. Regardless of your other responsibilities
and titles, because you possess the right mix of skills and experiences to best help and advance the
research program at your facility.
Help Resolve Any Issues That Arise
One reason why AOs are so busy is because they are the “go to” individuals for any problem that arises
at their facility. If you have an open-door policy, you will need to be able to handle frequent
interruptions. Your ACOS may have many other duties, including clinical care, university commitments,
lab supervision, executive management level meetings, and so on. This will force the ACOS to be out of
the Research Office, from time to time.
You do not need to be the expert on everything, but one skill that is important to develop – as soon as
possible – is the knowledge of who to go to for what. Drilling down deeper into this concept, you need
to be aware of the pitfalls. You may find that some people steer you in the wrong direction, give you
partial information, or, otherwise, complicate or compromise your ability to resolve a problem. You will
build your team of trustworthy agents, who are willing and able to readily resolve whatever problems
arise. It helps to build commitment by continually reinforcing the value of research to Veterans among
your co-workers across departments.
Committees
The AO (or delegee in larger programs) usually serves as an ex officio, non-voting member on all the
Research oversight committees, such as:
• Institutional Review Board (IRB) – human subjects research (VHA Directive 1200.05)
• Institutional Animal Care and Use Committee (IACUC) – animal research (VHA Handbook
1200.07)
• Basic Science Review Board – neither animal nor human research (established as some facilities)
• Research and Development Committee (R&DC) – premier oversight body; sometimes acts as
basic science review group (VHA Directive 1200.01)
• Subcommittee on Research Safety Committee (VHA Directive 1200.08)
• Institutional Biosafety Committee (IBC) – reviews research involving recombinant DNA.
It is important that the AO understand that their involvement is to help the committee members with
their assessments, and not to drive towards a decision of their liking. The AO, like the RCO, should act as
an information provider and reluctant advisor in the committee setting. The AO will often report a status
update to various committees on issues impacting their committee or the research program. The RCO
and AO should work together to help the committees have all the information they need to make
accurate decisions and determinations.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 90 of 149
The AO usually also serves on other committees which may be established such as:
• Research Space Committee
• Research Budget or Finance Committee
• Research Security Committee.
The AO may also be appointed to a variety of medical center committees due to the impact those
committees may have on research operations or vice versa. Some medical center committees that may
require AO involvement are:
• Environment of Care
• Green Environmental Management Systems (GEMS)
• Administrative Officer Council
• Facility Space Committee
It is important to use these opportunities to build relationships with staff members from different
departments, throughout the medical center. You can call on these individuals to assist you in a variety
of situations. An additional “bonus” of these relationships is having contacts, outside of research, that,
should the need arise, can assist you.
The ACOS/R&D serves as the non-voting “Executive Secretary” of the R&D Committee. In this capacity,
the ACOS/R&D can brief the R&D Committee on such items as the ORD Field Conference Call, the Field
Research Advisory Committee (FRAC) meetings from minutes received from those meetings and other
goings-on in ORD. In addition, the ACOS/R&D has specific responsibilities outlined in the Office of
Research Oversight Facility Director’s Annual Certification that can be communicated to the R&D
Committee. These items include:
• Ensuring that all requests for research WOC appointments are appropriately justified and the
appointments comply with all applicable research, human resources management, and other VA
policies
The ACOS may also use the R&D Committee venue to have short presentations of various educational
topics that would be cogent to committee members regarding their responsibilities or responsibilities of
Principal Investigators set by either ORD and/or the local level.
Customer Service
The Research Office serves a variety of “customers.”
Principal Investigators and their staff come to the administrative office to process paperwork, obtain
information, seek help and guidance, as well as to resolve problems. They usually do not schedule
appointments in advance; they come as time permits.
With facility departments, such as IT, Facilities Management (Engineering), Financial Management
(Fiscal), Environmental Management (Housekeeping), and HR, Research Administrative Office is the
central entity in the struggle to get things done (as well as interfacing with those services). Occasionally,
there are inspections or reports that must be fulfilled – and need the cooperation of research to do so. It
is then, they become our customers, or we become their customers.
Much like the front desk at a hotel, a myriad of people – most unannounced – come to us needing some
sort of assistance. It is important to create a friendly, competent, and professional office environment.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 91 of 149
Working with Clinical Services
There are occasions when a research project requires the assistance of clinical services support. Most
commonly, such clinical services are Imaging (Radiology and Nuclear Medicine), Pathology, and
Laboratory Medicine. However, other clinical services, such as Pharmacy and Nursing, may also be asked
to assist with a research project. Assistance with a research project may take the form of additional
laboratory or imaging tests that are more than standard of care, or the use of a clinical service
employee’s time to be involved in a research study. Laboratory and/or imaging tests that are generally
requested more frequently than standard of care (blood tests, X-rays, CT, or PET scans, etc.), or a special
procedure, such as additional non-invasive testing (ECHO and EKG) or invasive procedures, including
endoscopies, vascular catheterizations, and nerve conduction studies (again more than standard of
care), incur costs and these costs must be reimbursed to the Medical Care appropriation.
An initial discussion with the Service Chief, or designee of the clinical service from which Medical Center
support, should first occur to see if the Clinical Service can support that request. Acknowledgement by
the Clinical Service that a research project can be supported should be documented by an Institutional
Support signature or memo from the Clinical Service to the Principal Investigator, denoting the specific
extent of support to be provided. Remember that VA-funded studies should also have the support of
Clinical Services, if required. Requests for a Clinical Informatics service of the Medical Center to assist a
Principal Investigator to extract local data should also be approved by Clinical Informatics if sufficient
manpower is available to provide such data search and extraction.
Most Clinical Service Chiefs, who are academically inclined, welcome the opportunity to assist a Principal
Investigator, as this also may enable a Clinical Service Chief to introduce their faculty in perhaps
establishing an informal or even formal collaboration with other Investigators. Another incentive to
garnish support from a Clinical Service Chief is if reimbursements for clinical services that were provided
by that Service Chief’s Service, would go back into fund control point(s) specific to the Clinical Service.
Thus, when Medical Care appropriation is used to assist a research project, reimbursement must occur
for services rendered that are over and above routine clinical care and purely for research purposes (38
CFR § 17.102). Reimbursements to Medical Care appropriation for clinical services or “reimbursables,”
can be accomplished by several mechanisms. After approval by the Clinical Service Chief is obtained,
charges for services rendered must be determined. Costs for services can be obtained from VA Decision
Support system, prevailing Medical Care Collections Fund (MCCF) rates, “Reasonable Charges”
Chargemaster (a national computerized listing of hospital charges for services and supplies, adjusted for
local costs), locally adjusted Medicare Rates, Champus/VA Maximum Allowable Charges (CMAC), or
even Local Clinical Diagnostic Laboratory Fee Schedule. Once determined how charges are to be set, the
charges should carry over for the duration of the study, as grants or contracts will have set fees that –
for the most part – cannot be changed over the length of the grant or contract. Current Procedural
Terminology (CPT) codes can be used to identify the type of service or test rendered. Once the cost basis
methodology is chosen, it may be worthwhile to develop a tracking system by the Principal Investigator,
Research Study, enrolled patient, and costs incurred per enrolled patient, if granular detail of
reimbursable is desired. An alternative system would be to calculate the reimbursable percentage of the
total subject cost (reimbursable costs/total per subject payment). Here, a total reimbursable
percentage is determined and for subjects who may not complete a trial, and incur only a fraction of the
total reimbursable, charges reimbursed per patient could be higher than actual.
There are also different paths to bill for charges incurred. The Medical Center Financial Management
Office, which has a stake in assuring reimbursement of Medical Care appropriation, could provide the
Bill of Collection. Alternatively, Research Service Budget Office may be the biller for reimbursables. The
organizations that can be billed would be those agencies administering non-VA grants and contracts that
used VA clinical services in support of the research grant or contract. These organizations are typically
the VA non-profits or the university affiliate. Receivables go to the Agent Cashier at the VA. It is helpful

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 92 of 149
that the paying institution be given on the invoice, the Clinical Service fund control point to which the
reimbursable should be directed so that the check payment clearly reflects this specific fund control
point, otherwise the reimbursable would go to a general fund control point of the Medical Center and
not get back to the Clinical Service that provided the support. A Standard Operating Procedure and
Memorandum of Understanding between the VA and Non-Profit Corporation and/or university affiliate
on how reimbursables will occur should be considered. NAVREF has published a guide on
Reimbursement of the Medical Care Appropriation (see Resources for NPC Managers at the NAVREF site
at www.navref.org).
VA employees whose main duties are in the clinical realm may sometimes be requested to be involved
to support a research project (e.g., nuclear medicine technician). A part-time VA paid employee can
perform the research duties outside of their tour of duty by being brought on as a Without
Compensation, or WOC, employee with clear delineation between their VA tour of duty and the VA non-
profit or affiliate tour of duty and be remunerated for services directly from the VA non-profit. A full-
time VA-paid employee must be paid overtime for time spent on assisting with the research protocol,
even if the employee’s time assisting a research project is outside of the employee’s tour of duty.
Research animal work can be performed in clinical settings on equipment that is required by the
protocol. Approval from the Clinical Service Chief is required. Generally, such use of clinical equipment
in a research animal setting is done after regular hours or on weekends/holidays. The Animal
Component of Research Protocol (ACORP, Appendix 7) covers use of animal research in clinical care
environments.
Research Space
An essential infrastructure component of a successful and vibrant research program is the procurement
of sufficient research space to support the various types of ongoing research activities. A rational
mechanism of space assignment, space renovations, assessment of the quality of research space, and
the review of space usage will facilitate the successful accomplishment of research projects and enable
for the growth of research programs that will lead to the enhancement of productivity and recognition
of the research enterprise.
Assignment and Review of Space
All space within the confines of the Medical Center belongs to the Institution, and its purpose and use
will be designated by the Director of the Medical Center. The Medical Center may have a Space and
Resources Planning Committee that may have responsibility for research space. In some Medical
Centers, Research Service is granted the ability to allocate space 1) to individual qualifying Investigators;
2) to groups of qualifying Investigators with common interests forming a research unit; 3) for core
facilities as designated by Research Service; 4) for the use by Research Service that would best serve the
interests of the Service, which may include space allocation for properly executed Sharing Agreements
benefiting Research at the Medical Center. If Research Service is delegated to handle Research space
matters, there may be a Research Space Committee that functions to distribute and review space, or the
R&D Committee may serve in that function in the absence of a separate Research Space Committee, or
in other instances, the ACOS/R&D may serve as arbiter of space distribution and review.
The space assigned to an Investigator will in general be proportional to the amount of funding support
or number of funded PIs who can share space with a similar research topic, however, the need must be
justified. The following criteria could be considered in space allocation:
1. Research Funding
a. Research programs funded by VA Merit Review Award, or similar VA funding.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 93 of 149
b. Peer-reviewed research with high priority or relevance to the care of Veterans supported by
extramural funds only and conducted by an Investigator who is eligible for VA funding.
c. Non-peer reviewed research supported by extramural funding with high priority or
relevance to the care of Veterans.
2. Common resource research activities (e.g., use of equipment, research techniques, or focused
interests as established in a formal Core Facility or other arrangement with several
investigators).
3. Productivity: This will be judged by publication of substantive papers in critically reviewed
journals. It is recognized that mere numbers of publications will not necessarily establish a high
degree of priority, but rather, the significance of the work (as evaluated by ad hoc reviewers, if
necessary, as well as the professional stature of the journals).
Clinical relevance of the Investigator: This factor will be based on an Investigator’s primary clinical
responsibilities (e.g., recruitment/retention of rare or selected critical specialties).
Considerations other than outright assignment of Research space, but having space “loaned”, could
include the following criteria:
1. Newly recruited Investigators who have no funds, but are eligible for VA funds, and have applied
for VA funding, or are in the process of doing so.
2. Temporary expansions in space for ongoing programs.
3. Research programs which are not funded but are being performed by VA personnel who have
been determined clinically indispensable.
4. Programs that support important clinical functions.
Generally, space is committed to an Investigator for the length of the funding of the specific program.
Career Development Awardees (whatever the source of funding) are usually housed in space of their
mentor(s). Career Development Awardees transitioning from trainee status to independent status can
be “loaned” space until it is deemed by the R&D Committee that independent funding for a research
program has been established.
Office space for Clinician Investigators/Non-Clinician Investigators may be scarce, depending on the
facility. However, it should be noted that some research programs, such as health services research and
clinical research highly depend on office space to carry out the research. Careful consideration should be
given when there is thought to convert “wet” laboratory space to office space, unless the need of
emerging programs requiring office type research space is a high priority.
Review of research space is suggested to be conducted on an annual basis. Useable research space can
be reallocated. In addition, the following considerations can be taken into account (not necessarily in
the order of importance):
1. Value of the program and Investigator to the VA
2. Impact of space reallocation on other Investigators
3. Impact on collaborations, core facilities, equipment, etc.
4. Personnel versus equipment requirements of the project
5. RDIS II Report of most recent reported research expenditures
6. History of grant/contract funding and proposal submission. Investigators without currently
funded grants for a pre-determined period (e.g., 2 or 3 or more years) since their last funding
period could be in jeopardy of having space reclaimed. Investigators without grant funding for a
predetermined period, but who have continued to submit research proposals for funding
consideration may be loaned space or could need to share the present space with other
Investigators.
7. Publication history
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 94 of 149
8. History of having non-VA grants/contracts administered through the VA non-profit corporations
may be given preference for VA research space
Review of space for Investigators involved in Health Services Research and Centers (including
Rehabilitation R&D Centers) has some special caveats. Health Services Research and Rehabilitation R&D
space may be under the auspices of a local HSR&D COIN Rehabilitation Center/REAP, and respectively,
and generally, space distribution for such investigators is governed though the COIN or Center/REAP.
When space needs to be reassigned from one Investigator to another, mechanism(s) should be put in
place for appeal of the space reassignment should the Investigator losing assigned research space have a
valid reason for continuing to be assigned space.
Renovations
Requests for research space renovations should be detailed in memorandum form addressed to the
R&D Committee or ACOS/R&D. The following information is suggested to be included in the request: a)
type and extent of renovations, b) purpose of renovations and/or justification of renovation, c)
Investigator affiliation and time commitment to the Medical Center (i.e., VA, University, WOC, percent
VA time, etc.), d) cost of renovations, e) whether renovations are to be done by independent contractor
furnished by the Investigator or by VA Facilities Management Service and/or VA-delegated contracting
service, f) if current non-VA grants/contracts are administered by VA non-profit corporations.
1. For renovations to be financed by the Investigator or group of Investigators and performed by
private contractor, concurrence with Facilities Management Service must be obtained.
2. For renovations to be funded by the Medical Center, projects will be prioritized by the R& D
Committee or ACOS/R&D with input as necessary from the Subcommittee for Research Safety
(SRS) and the Institutional Animal Care and Use Committee (IACUC), according to the following
suggested guidelines:
a. Renovations necessary for safety and/or health issues deemed urgent by the SRS and/or the
Medical Center or renovations required for accreditation.
b. Research core facilities
c. Faculty recruitments
d. Investigator group renovations that would enable close collaborations
Appropriate justification with documentation as to the reason for renovation must be included in the
renovations request.
The routine maintenance of electrical, plumbing, air-conditioning, and heating repairs are to be directed
through the ACOS/R&D or Administrative Officer or other appointed Research Service employee
responsible for work orders. Only where there is construction, new wiring, new plumbing, installation of
lab benches, etc., should requests be made through the R&D Committee and/or the ACOS/R&D.
In general, renovations or the scope of a project that falls outside of the Maintenance and Operations
Office of Facilities Management is delegated to the Projects section of Facilities Management. These
projects could be Non-Recurrent Maintenance projects, Minor Construction projects, or rarely, Major
Construction projects (this latter is generally funded only rarely).
3. Information regarding prioritization of renovations projects should be made available to all
Investigators. Follow-up of progress of approved renovations can be done through reports given
by the Projects Section, Facilities Management and/or Maintenance and Operations, Facilities
Management.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 95 of 149
Tips in Space Management
1. Have a transparent process for space assignment and review. It is suggested that either the
Research Space Committee or R&D Committee handle research space matters.
2. Have a formal application form for all space requests, and clearly written Standard Operating
Procedures on space assignment, review, and renovation with specific criteria that are clearly
delineated and prioritized.
3. Should research space not be available on the VA site, consideration can be given to leasing
space to perform research (perhaps more facile for research needing office space). Currently,
ORD has been working with the VA’s Real Property Office and VA Counsel to put a process in
place when VA investigators occupy non-VA space. A template for VA use of non-VA space
would need to cover the current off-site waivers approved by ORD. More information to follow.
General Oversight of Research Lab Areas and Other Odds and Ends
The Administrative Officer is usually an important figurehead for management of the research lab area.
It is a good practice for the AO to walk through the lab areas regularly and check in with lab staff to
ensure that everything is going smoothly. The responsibilities associated with lab security can also be
assessed while out in the lab area. The AO should be knowledgeable of basic lab safety and security
criteria and help identify potential issues before they become a problem or are cited in an inspection.
They should be able to guide lab staff to appropriate people for assistance outside their own expertise.
The AO should understand the needs of the lab area and help plan for future needs. They should also
have a basic understanding of the Biosafety in Microbiological and Biomedical Laboratories document
(BMBL), which can be found at http://www.cdc.gov/biosafety/publications/bmbl5/.
Enter Work Orders to Engineering
Your facility will have a system in place to notify Engineering of issues that require attention. Anyone
with access to VistA, or whatever system the facility uses, can enter a work order to the Engineering
department. The AO is usually the work order approver.
Many researchers would rather just call the AO, inform them of the problem and have them take it from
there. It is important to get as much detailed information, as possible, so that you can convey the
problem properly to Engineering. Therefore, it is essential to become familiar with the local work order
submission requirements, so you can submit complete work orders, and insure the prompt correction of
the problem.
Engineering should provide Research with contact numbers for emergencies. Off-hour emergencies may
be handled through the Medical Center Police.
Minor issues – like fixing a light that has burned out or replacing a broken ceiling tile – are sometimes
handled by a “Cart Man”, or person who roams the facility repairing minor fixes.
Examples Engineering Work Orders:
• Install a rack for gas cylinders
• The eye wash is starting to come off the wall
• A new piece of equipment needs to be installed with appropriate power supply
• There is a slow leak in the restroom
Involvement in Emergency Preparedness Plan
The Research Service should have its own emergency preparedness plan, and the AO should be a part of
its creation and maintenance. It is probably good practice to integrate the Research Emergency
Preparedness Plan into the Medical Center’s overall Emergency Preparedness Plan. This can be done by
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 96 of 149
working with the Medical Center’s folks involved with this; many times, the Industrial Hygiene or Safety
Office takes the lead with the Facility’s Emergency Preparedness. It is important that this information be
shared with researchers who work in dedicated research areas, so that they know what to do and where
to go in the event of a crisis.
It is the responsibility of the AO or other designee to ensure that emergency drills are conducted. These
drills can occur at the direction of the facility Safety Office, Subcommittee on Research Safety, or the
Research and Development Committee.
Emergency Cascade Plan
Every service at your facility has an Emergency Cascade Plan. This is a document that lists all the
members of the service with emergency contact information. The person at the top of the list calls the
person next down on the list, that person calls the person below them, and so forth. It launches from
the ACOS/R&D, who will provide any information and instructions. The Emergency Cascade Plan should
be updated, as needed and kept current. You may need to send a copy to the facility Safety Office, or
other designee. There are commercial companies that assist to organize the cascade to make it easy to
“spread the word.”
Additionally, it is helpful to send an electronic copy of this document to your personal email address, so
you have access to the information even if you are away from home or the facility.
Facility Level
Usually the ACOS/R&D is part of the facility emergency response team. Sometimes, the AO is part of this
team as well. Your local facility will inform you regarding what is expected of you and the Research
Service team, in the case of emergencies.
New Staff
When new staff are appointed to the Research Office, the AO or designee is responsible for ensuring
that the person is properly credentialed, has a Scope of Practice, and is appropriately tied to a
laboratory, project, or administrative entity. The research office will assist in obtaining that person’s
office or desk space, including a telephone, IT equipment, supplies, badge, access to IT systems, and
orientation. Depending on your location, you may need several weeks’ lead time to set up a new
employee with needed resources.
As stated earlier in this document, PIs are responsible for their new staff members’ personnel
requirements, but may need assistance from the Research Office for some additional elements.
PIV Badges
The Personal Identification Verification, or PIV badge features an embedded gold microchip that
contains information about the badge owner. The process to get one of these badges involves getting a
VA email address and having the person setup in the PIV portal by their “Manager.” Then the “Sponsor”
must enter the PIV portal to add additional information. In some cases, the Manager and Sponsor can be
the same person. After completing this process, the person is ready to be finalized in the system by the
Badging Office (depending on the station, it could be HR or Police Service), and is issued a badge. This
process must be coordinated with HR, so that security check clearance is underway before the badge is
issued. At some facilities, the AO acts as the PIV Portal “Manager” and “Sponsor.”
Service Needs
The AO can also be an office manager, ensuring that the Research Office has the resources it needs in
the way of personnel, space, furniture, copy machines, printers, telephones, audio/visual equipment,
and supplies. Typically, all these items should be procured through the local VA facility, as these items
are not specific to a research study.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 97 of 149
The AO is involved in the planning and management of the common areas within research areas,
including clinical, lab, and VMU for furnishings and common equipment. The AO often assists
researchers, helping them find the items they need for their research areas, subject to the AO’s
expertise.
Oversight of Construction and Upgrade Projects
From time to time, your facility may receive funds for construction or upgrade projects that involve
research areas. The AO will be an important part of the planning and coordination of these projects. As
the AO has knowledge of the researchers’ needs that surpasses that of any of the contractors or
engineering liaisons, and it is important to help them understand those needs.
Communication during construction and upgrade projects is very important. The AO or designee will be
the point of contact for the contractors, and engineering liaison for any issues that come up, so that you
can forward any information that the researchers might require. (The researchers should know to notify
the AO, if they have any problems or concerns.)
The involvement of the AO and R&D office is critical during construction and upgrades, especially when
standard services are going to be electively terminated. It is not uncommon for engineering
professionals in the HVAC, plumbing, or electrical shops to coordinate tests or upgrades of their systems
to minimize or eliminate any negative effects from a planned utility system shutdown. Sharing this
information with researchers in advance is essential.
Key Requests
The Research Office should have a Security Plan in place to control access to research areas. Sometimes
researchers change location, or when a new researcher comes on board, they will need keys or access
badges to their areas. The AO (or the AO may delegate to someone in the research office) may be the
person to approve and make the request to the group in the facility responsible for issuing keys. There
may be research training requirements that need to be met before keys should be issued.
Sponsoring key requests for WOC employees may be a hurdle if the sponsor is not a VA-paid employee.
This may be true with a WOC Principal Investigator located at the VA who also has WOC employees in
his/her research program. In those cases, another VA-paid Investigator may want to sponsor the key
request especially if the WOC researcher is also collaborating with the VA-paid investigator. In more
times than not, the Research Office may need to sponsor the key. If that is the procedure followed, the
Research Office needs a mechanism to track which WOCs have been issued keys so that returning keys
can be made part of the clearance process when WOCs leave the Service.
The AO, as well as the ACOS/R&D, should have a master key to all research areas.
Hood Certification
Fume hoods and biological safety cabinets need to be certified at least annually. Safety cabinets in BSL3
facilities must be certified semi-annually. The AO or the Research Safety Coordinator, through the
Research Safety Committee, may be responsible for ensuring that hoods are properly certified. It is also
possible that this function has been assigned or delegated to local engineering personnel or the
Industrial Hygienist, so it is very important that you confirm the local process for hood certification.
Prescription Pads for Research MDs
From time to time there may be a physician who works only in research who needs prescription pads.
These government prescription pads are numbered and not pre-printed with physician information.
Someone in your facility is responsible for issuing them and they will want to issue them to the service
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 98 of 149
AO. You will need to keep these in a secure location and document to whom and when they were issued
by you.
Staff Meetings
It is a good practice to conduct regular staff meetings for research administration staff. You may or may
not want to include the staff of the VA Research non-profit. Your ACOS/R&D may want to participate or
even lead the meetings. This is a good opportunity to share news from the facility, update staff on
recent events, clarify issues that have become “confused”, as well as share information obtained at
training and discuss new policies.
Be careful not to let these meetings become forums for public attacks on any one staff member, as
these issues should be handled in private and between the people involved.
Also, your staff will appreciate it if you keep the meetings organized and time sensitive.
Researcher Communication and Meetings
AOs should have ways to communicate with different groups of the research community. You may set
up an email distribution list for all research personnel, research investigators, or other important
subgroups that you need to reach for important notifications. This can be done through IT with a
network distribution list, or you can create your own distribution list in MS Outlook Contacts™.
It is also a good idea to have regular meetings with researchers. Some of these meetings may be
scientific in nature – the sharing of ideas, creating a spirit of collegiality and collaboration. These
“meetings” can also be virtual with a periodic Newsletter or other type of electronic communications
sent out on important information items for Investigators and their research staff.
Other meetings may be regarding new regulations or changes to SOPs. Meetings could be targeted to
research coordinators, research assistants, lab techs, PIs or any combination of these groups depending
on the need.
Research Week Event Planning and Coordination
Every year ORD announces Research Week, usually in April or May. ORD strongly encourages each
research program in the field to have its own local events and invite the local community and leadership
to the event.
An event like this might have the following agenda at your facility:
• Poster presentations
• Leadership Welcome
o Director
o Chief of Staff
o ACOS/R&D
• Veteran Panel
o Veteran experience with Research
• Keynote Speaker
• Q&A
• Food & Beverage/Poster Review
You should coordinate your event with your facility Director, Chief of Staff and local Public Relations
Coordinator.Have the materials that are sent to you from ORD posted throughout the medical center.
(Is there a marquis at your facility? Post event information on the marquis if possible.)

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 99 of 149
SECTION 11 – Information Technology (IT)
IT Allocation – Attention to Source of Computers and Data Storage Devices
Perhaps one of the more complex and complicated aspects of administering a research program is
dealing with items related to Information Technology (IT). Administrative computers for Research
Service and those computers for Investigators and research staff that are to be connected to the VA
network should be obtained from the local IT Service.
Funding
VA Directive 6008, “Acquisition and Management of VA Information Technology Resources” defines
what VA IT or non-VA IT assets can be purchased through different funding sources (e.g., Research, IT,
Non-VA Funding). Computers and other data storage devices can be purchased through the university
affiliate, the VA non-profit corporation, or other institutions or entities that may support the research to
be done at the VA.
Thus, there are two types of VA funding for IT as it pertains to Research. Your local IT department is
responsible for fulfilling all general (non-project specific) IT needs of the Research Department. Standard
leased PCs, printers, network connections, and most software (Operating System, Office Productivity
software (such as MS Outlook®, Word®, Excel®, PowerPoint®, and Adobe® Acrobat) are to be provided
by the facility IT department.
Software that is uniquely used for research may be purchased with research funds. Examples might be
software to analyze images. Statistics software does not meet this requirement as it is also used by
other portions of the VA.
Aside from hard IT equipment, computer general software also needs to be purchase through IT
appropriation. With regards to guidance on what can and cannot be purchased from IT or non-IT
funding, the Director, Bioinformatics, ORD, has developed a Frequently Asked Questions.
IT Equipment Requests
For basic IT Services (e.g., desktops/laptops, printers, wireless cards, smartphones, help desk and
telecommunications support) OIT’s “yourIT” ServiceNow p
rovides the capability to submit a request for
these types of IT equipment requests. Through this submission process, your local IT department is will
provide basic IT Services (e.g., standard PCs and computing equipment, printers, portable/removable
storage devices, and monitors) for general, networked, non-scientific use. The IT department at your
facility will advise you of any additional processes for submitting new IT Equipment and/or replacement
requests.
Research Scientific Computing Devices (RSCD)
Some software and IT equipment can be purchased with VA research funds. Computers that operate
research equipment are considered part of that equipment and can be purchased with research funds.
OIS classifies these types of equipment as a Research Scientific Computing Device (RSCD). R
SCDs are
usually not loaded with VA security features or encryption as this would interfere with the software
running the equipment. OIS has developed an enterprise process for securely connecting a RSCD to the
VA Network for appropriate research purposes. Researchers and Research Staff are encouraged and
recommended to review the requirements for submitting a RSCD for connection to the VA Network
through the Enterprise Risk Analysis (ERA) process at ERA Portal. A
dditional training on the process is
available at the RSCD ERA Training Resource Guide. Through the ERA process, RSCDs will be logically

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 100 of 149
isolated on the VA Network through a Device Isolation Architecture (e.g., Virtual Local Area Network
(VLAN)).
IT/RSCD Inventory Management
All equipment maintained in VA facilities is required to be inventoried. Even if the VA does not own the
equipment. Research devices and/or RSCDs are often donated or acquired using non-OIT funds and are
supported by the scientific team making the acquisition. This support may include patches to operating
system, upgrades to firmware or application layers, and management of data including backup and
restoration. The use of personally owned information systems on-site to perform assigned official
duties must be approved by the Area Manager/System Owner or designee.
VA Handbook 7002 states, “The VA CIO is responsible, at the Department level, for ensuring the integrity
and security of VA’s IT assets, including physical inventory as well as data protection and the sanitization
of data when IT resources are retired from service.”
VA Handbook 7002 states, “Equipment owned by an affiliated institution, or purchased by such
institution from grant funds, used by a VA investigator in a research project at a VA installation will be
accounted for in the appropriate VA property accountability system, regardless of cost of the
equipment.”
• OIT maintains the Automated Engineering Management System/Medical Equipment
Reporting System (AEMS/MERS) as well as MAXIMO for this purpose.
• OIT Owned: All hardware in this basic & advanced infrastructure stack should be owned by
OIT and recorded in an Equipment Inventory List (EIL) Managed by the local area manager.
Since all the Lifecycle Management funds (money available to replace the oldest hardware
first according to industry accepted standards) is distributed by OIT based on information on
equipment age from the EIL.
• Equipment purchased by ORD using funds from their non-profit corporation (NPC) should be
donated to the VA and listed on the OIT EIL for that medical center. (See discussion of
“Scientific computing” below for exceptions to this practice.
• Equipment not owned by the VA and purchased by an affiliate institution and used at VHA
facilities need to be entered into the EIL.
o Note: equipment on a EIL does not indicate VA Ownership.
• Annually, a physical inventory of all nonexpendable accountable property and designated
sensitive items will be conducted. (VA Handbook 7002 8.5).
Inventory Best Practices
• There does not have to be just one EIL.
o Group devices in a useful manner on their own EIL.
• Document a clear Standard Operating Procedure for inventory processes
o Make it accessible for all staff
• Identify a single point of contact to coordinate research inventory for your project.
o When inventory becomes a standard practice, it is easier to maintain.
• Make sure the team has a way to input equipment into inventory.
o Create an Excel spreadsheet with requisite data for input to the database
• Make it easy to identify equipment.
o Colored labels a
re a great visual indicator, choose any number of color combinations
to indicate Research equipment, affiliate owned, or even equipment entered in an
EIL.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 101 of 149
o Colored labels do not have to be on the equipment – Being visible nearby works as
well.
It would be preferred to involve the VAMC clinical engineering staff in support of many RSCDs or for
research to obtain a support contract for their maintenance, troubleshooting, and problem fixing.
Examples such as large neuroimaging research centers may require considerable clinical engineering
support for routine maintenance, troubleshooting, and storage/backup of data.
An excellent ORO Checklist is available at the ORO website on Information Security program matters
detailing what requirements need to be fulfilled (https://www.va.gov/ORO/orochecklists.asp
)
VHA Research and Development IT/Informatics Guidance FAQs (Updated
Annually)
ORD disseminates a FAQ annually to the research community. This FAQ contains additional guidance
around the acquisition and management of VA Information Technology Resources, IT Budgeting,
requesting VA OIT services, scientific computing, statistical analysis, and research analytical tools. It also
includes guidance around inventory management and Enterprise Risk Analysis (ERA) process
requirements for Research Scientific Computing Devices (RSCD). The FAQ can be found at
https://www.research.va.gov/resources/policies/guidance/ORD-IT-FAQs-and-Guidance.docx
Additional FAQ and other Research IT/information security guidance resources can be obtained through
Research and Operational Technology Cybersecurity Division (ROTC-D). ROTC-D consist of a specialized
team of cybersecurity specialists and enterprise ISSO staff dedicated to supporting the VA Research
mission for research information security/cybersecurity related issues. A link to ROTC-D guidance
resources and FAQ can be located on the Guidance Resource Portal.
Other resources include the ORD Research Information Security & Cybersecurity Toolkit.
These resources offer a variety of research information security/cybersecurity related guidance.
Questions for research information security support services can be requested via VHA Regulatory
Research Cybersecurity Guidance ServiceNow Catalog in “yourIT” ServiceNow. Additionally, ROTC-D
can be reached via OIS ISPS SDCD Research & Operational Technology Cybersecurity Division.
Remote Access to the VA Network
Researchers may work off the VA site yet need access to the VA network where VA research data or
other research-related documents, images, etc., are stored. To request remote access to VA systems, go
to the Remote Access Portal (https://vaww.ramp.vansoc.va.gov/Pages/Dashboard.aspx) and complete
the application.
ADPAC – Automated Data Package Application Coordinator
As AO, you may be the ADPAC for your service (also known as Technical Application Coordinator at some
facilities). This person approves IT equipment requests, approves requests for data storage space on the
shared network hard drive, and is the lead IT liaison for IT rollouts of new software and procedures.
Information System Security Officer (ISSO)

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 102 of 149
Also, mentioned in the IT section above, the ISSO is the person at your facility that serves as your
primary point of contact for research related information security questions/concerns and data security.
This critical position tells us how to protect what needs to be protected. The ISSO will review studies
that involve the collection, processing, storage, and transmission of research data to ensure that the
proposed research complies with information security policy and that study data is managed, according
to VA regulations. The ISSO will resolve any questions or concerns related to a study’s data security plan
directly with the researcher submitting the study.
As you know from your VA Privacy and Information Security Awareness and Rules of Behavior, you are
required to report suspected or actual information security and privacy incidents immediately to your
supervisor, ISSO and PO. Notification of an incident to your ISSO will be reported to the Cybersecurity
Operations Center (CSOC) in accordance with VA Incident Reporting Policy. If the name of the ISSO or PO
at your facility is unknown, you can search the ISSO locator and PO locator. T
he incident should also be
reported to the ACOS for Research. This triggers the need for the Director to notify ORO of the incident
within 5 business days. See VA Handbook 1058.01 – Research Compliance Reporting Requirements for
more details. https://www.va.gov/ORO/oropubs.asp
Office of Information Security (OIS), Research and Operational Technology
Cybersecurity Division (ROTC-D)
Research and Operational Technology Cybersecurity Division consults with stakeholders across the VA
enterprise participating in research programs, providing guidance in complying with research
information security policy. The ROTC-D Team consists of cybersecurity specialists, Subject Matter
Experts, and enterprise Information System Security Officers who are responsible for responding to
enterprise cybersecurity requests and needs of VHA stakeholders through the development of research
information security guidelines, procedures, training, and policy. ROTC-D prioritizes the development of
enterprise cybersecurity processes and the adoption of approved technologies that align with the
business considerations and objectives of the Office of Research & Development (ORD) and the Office of
Research Oversight (ORO). ROTC-D can be reached via OIS ISPS SDCD Research & Operational
Technology Cybersecurity Division.
Privacy Officer
The Privacy Officer (PO) is responsible for ensuring that patient information meets all privacy
regulations. This critical position tells us what needs to be protected. This person is responsible for
HIPAA documentation. The PO serves in an advisory capacity on the IRB or R&DC as ex-officio non-voting
member or consultant. (See VHA Directives 1200.01 and 1200.05 for more information). They also
review all human subjects’ projects at the time of initial review to ensure that all required privacy
protections are in place. He or she must document the review and receive a copy of the approval
paperwork once the project receives final approval. You can identify your local Privacy Officer by using
the PO Locator.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 103 of 149
SECTION 12 – Project Management
As AO, you will find that some of your work will entail executing a multi-faceted project involving several
people performing various tasks over time… project management. It is helpful to have a grasp on a
system that will help ensure that the project you are leading is progressing as needed. VA Acquisition
Academy offers courses in project management leading to certification. Information is available at:
www.acquisitionacademy.va.gov
Compliance Issues
For example, after an inspection, a compliance finding may have been identified that requires
researchers, engineering and/or fiscal to perform tasks. You will need to track everyone’s performance
in correcting the finding, as to report to the oversight body within the given timeframe. Within some
research programs the RCO handles these matters; at others, it is the AO or designee.
Tracking Dates
There are many deadlines within the VA and the AO needs to be on top of them and enable enough time
for others to complete their portion of the project. A general calendar of some of these items can be
found in Appendix C.
Liaison for Construction and Engineering Projects
As mentioned earlier, the AO or designee will be the primary point of contact on construction and
engineering project involving research areas. The AO needs to be made aware of the timeline and scope
of these projects with frequent updates regarding delays, problems or completion. The AO should
communicate important and relevant information to researchers.
Paperwork Reduction Act
The Paperwork Reduction Act defines when collections of information require OMB clearance. VA
Directive 6309: Collection of Information and VA Handbook 6309: Collections of Information Procedures
describes VA’s policies for compliance with the Paperwork Reduction Act. VA Handbook 6309 describes
items considered not be collections that should generally not be information defined under the
Paperwork Reduction Act, collections of information not subject to the Paperwork Reduction Act, and
collections of information subject to the paperwork reduction act. All collections of information do not
require OMB clearance, but when the collection of information requires OMB clearance, it must be
obtained. For example, all qualitative activities (both research and non-research) do not require OMB
clearance
when the information is not obtained by means of identical questions or identical
reporting, recordkeeping, or disclosure requirements. For those that do, obtaining clearance is
required.
For ORD funded studies, such as the HSR&D and CSP studies, the funding service as a process
for evaluating clearance and works with the VHA PRA office for the studies they fund when the
collection of information requires OMB clearance. When there is an issue of whether the activity
requires OMB clearance, whether it be a research or non-research activity, the query should go to the
VHA PRA liaison at the VHA PRA shared mailbox at [email protected].

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 104 of 149
SECTION 13 – Compliance
There are many regulations affecting VA research in the form of VHA Directives, Handbooks, Policies,
Federal, State and local laws as well as accreditation organizations. Compliance with these regulations is
primarily the responsibility of the Research Compliance Officer (RCO) but a close relationship with the
ACOS/R&D and the AO makes this essential aspect of research very important.
All repetitive activities, including regulatory requirements should be documented in local policies or
SOPs as outside regulatory agencies and accreditation review teams look for the local SOPs to see if
statements made within the SOPs are being adhered to.
ORO
ORO (Office of Research Oversight – https://www.va.gov/ORO/index.asp) is the primary internal VA
entity that ensures that every VA Research program is compliant with regulations. ORO reports to the
Under Secretary for Health.
ORO is organized into subject matter groups:
• Research Safety & Animal Welfare
• Policy & Education
• Human Research Protections
• Review Management and Integrity (see Section 30, Research Misconduct)
• Research Information Security
• Informatics & Data Analysis
Your MCD submits a report to ORO every year: The Facility Director's Certification of Research
Oversight. Instructions are posted on ORO’s website (https://www.va.gov/ORO/index.asp
). In
formation
to complete the form will come from various sources including ePROMISe, Committee records, audit
records held by the Research Compliance Officer.
ORO also conducts site visits. Their goals are:
1. Conduct a standardized review of each facility’s infrastructure for protecting human subjects,
investigators, and animals in research at least every fifth year.
2. Regularly scheduled Comprehensive Program Reviews (CPRs) (every 5th year) Virtual and
Limited Onsite Components
3. Holistic Integrated Review: R&DC and Research Admin, HRPP, ACUP, RSSP, and RISP
Streamlined Report to Facility
4. Follow-up reviews based on Prioritized Area(s) of Vulnerability Focused Onsite Reviews
Onsite Technical Assistance Reviews
5. Remote Follow-up Reviews
6. Continue For-Cause Onsite Reviews, as needed
ORO has developed many checklists that help identify and assess local compliance with requirements.
They may be found at https://www.va.gov/ORO/orochecklists.asp. M
any other entities will inspect or
survey the program for compliance with their regulations (e.g., FDA, AAALAC, OSHA, ITOC, OIG, etc.).

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 105 of 149
RCO
When the Research Compliance Officer (RCO) reveals a compliance issue he or she may take it up with
the AO, ACOS/R&D, or other administrative sections in Research Service to correct the problem. There
may be reporting requirements.
13
The RCO reports to the MCD. Research compliance works best when
the RCO and the Research Office work together to identify and correct problems, as they arise and
educate investigators how to do the right thing.
Corrective Action Plan
As AO, you need to understand the compliance issue and verify that it really is a compliance issue. There
have been instances where an inspector or surveyor thought something was a regulation that was not.
Moreover, directives and handbooks are revised on a regular basis. What may have been a discrepancy
six months ago may no longer be out of compliance. Once all agree that there is a compliance issue, the
AO is part of the team that helps come up with the corrective action plan. Wherever possible, correct
the non-compliance on the spot. If the matter can’t be rectified in the moment it is identified, then a
corrective action plan will be needed. Depending on the inspecting entity, this plan may need their
approval before implementation. The corrective action plan may be simple or complicated; it may take a
short amount of time or a long time. The AO needs to track it and ensure that it is accomplished.
13
See VHA Handbook 1058.01 for more on ORO reporting requirements

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 106 of 149
SECTION 14 – Updating Databases
ePROMISe
ePROMISe ([enterprise] Project Management & Information System) is the database used to inform ORD
of all approved research protocols and investigators. It is linked to the VA National Headquarters R&D
Computing Center (RDCC) which is responsible for maintaining the VA Research and Development
Information System (RDIS) and ePROMISe. You can pull forms from this system that will provide you
with information needed to properly enter your studies, such as the funding source codes and
administrative codes. Every station has one or more local Administrators who can create local user
accounts and reset passwords. If a new Local Administrator account is required, the requestor needs to
first obtain local AO approval and communicate with the RDCC via a NSD ticket thru yourIT (icon located
on VA desktops). RDCC will create a new Local Administrator account. There are no account request
forms for ePROMISe. Please request any NSD tickets being opened to be assigned to the ‘ITS ORD SW’
group.
RDIS – Accessed thru ePROMISe
RDIS (Research and Development Information System) is also maintained by the
Research and Development Computing Center (RDCC). Information from RDIS is sent to
the Allocation Resource Center (ARC), to determine the allotment to each facility
(through the VISN) of VERA (Veterans Equitable Resource Allocation) dollars. Research
VERA is shown in the VERA reports under the ARC website VHA - ARC (va.gov)
VAIRRS (VA Innovation and Research Review System)
VAIRRS is the VA’s enterprise instance of IRBNet. VAIRRS is the research committee software
management platform for all VA medical centers with research programs and is uniquely positioned to
withstand the changing pressures of research needs, including:
• oversight needs of the research programs and institutions
• collaborative needs of dually appointed VHA investigators
• regulatory changes of the new Common Rule (2019)
Key benefits of VAIRRS include:
• reduction of administrative load on Research Office staff, committee members, researchers
and their project staff
• improved transparency of research protocol processing
• support for all committee work and allow for institutional tracking of all research and
innovation project regardless of funding or regulatory status
• dashboard reporting of key metrics; and harmonized and standardized processes across VA
research.
15

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 107 of 149
As of 2022, VAIRRS has been rolled out to all active research programs. While there may be programs
that continue to use other local committee management systems, all study actions are facilitated by
VAIRRS. For additional guidance, email [email protected] or the VAIRRS website. [email protected].
SECTION 15 – Affiliate University
One of the four statutory missions of the VA dictates that it conducts an education and training program
for health professions students and residents to enhance the quality of care provided to Veteran
patients within the VHA. Many VA Medical Centers have medical school affiliations with accredited
medical schools and osteopathic medical schools for physician education. Additionally, VA medical
centers have affiliation agreements with number of colleges and universities representing 40 other
health professions. In 2010, over 115,000 trainees received some or all their clinical training in VA
medical centers.
14
Does your VA medical center have its own IRB, or does it use the IRB of the affiliate medical school? If
the medical school IRB is the IRB of record for the VA, then it is required to follow all VA regulations.
Sometimes this is a challenge because VA regulations are sometimes more stringent than FDA or OHRP
regulations. The University “climate” may be more lenient than the VA environment and shifting to a
Veteran-centered consciousness may be difficult or challenging.
The AO or ACOS/R&D may be an ex officio, non-voting member of the university IRB. When ORO
inspects, it will review the affiliate IRB’s compliance with VA regulations.
Likewise, the affiliate university’s IACUC might be the animal committee of record for the VA medical
center. The roles of the affiliate IACUC is described throughout VHA Handbook 1200.07 and Section 8.b.,
8.c., and 8.d are devoted entirely to this relationship.
As of this writing, the VA is struggling to protect its government resources that can be so closely tied to
the University setting. PIs often have joint appointments – to the VA and to the University. Is it clear
what is VA space and what is University space? Is it clear who is a VA patient or a medical school
patient? Is the research VA research, university research or both? Who owns the data? How is the
researcher’s time split between the university and the VA? Collaboration with the university affiliate is
essential but it is fraught with challenges to protect the public interest in its funding.
Time Effort MOU Guidance
Background
Investigators with joint appointments at a VAMC and an affiliate university, who wish to apply for
funding from NIH, must have an MOU that defines their work/effort distribution at the two sites.
NIH requires that the MOU include:
• Title of the investigator's appointment
• Distribution of compensation
• Responsibilities of the proposed investigator
14
Office of Academic Affiliations website: http://www.va.gov/oaa/oaamission.asp
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 108 of 149
• Percentage of effort available for research at each institution with the joint VA/university
appointment making up 100% of the total professional responsibilities
• Signatures from the appropriate officials of the affiliate and the VAMC
Issues
Questions regarding the MOUs have been raised at various VAMCs. For example, it has been suggested
that the MOU, particularly when it includes salary figures, may appear to be a contract that is promising
a particular level of compensation. There has also been a concern that VA individuals who are signing on
behalf of the VA have a conflict of interest since they often also have a faculty appointment at the same
affiliate. Specifically, there has been a concern about ACOS/R&D and Service Chiefs who are often dual
appointees.
Investigators must be aware that NIH work done on VA time or in VA space is considered VA Research
and must be approved by the R&D Committee. Potential Conflicts of Interest must be reviewed to
ensure that the institutions and the PI do not run afoul of USC 209. More information can be sought
from the Office of General Counsel Ethics Specialty Team.
Plan
To assist the field, ORD proposed issuing guidance as to the preparation of the MOUs. It was felt that it
would not be appropriate or useful to develop a VA form for national use since the MOUs are typically
prepared by the affiliate in accordance with local preferences. Although NIH places the responsibility for
the MOU on the affiliate, it behooves the VAMC to ensure the document accurately reflects time and
effort at the VA.
Guidance for MOUs:
1. MOUs should reflect the time distribution between the affiliate and the VAMC along with the
percent of time available for research.
2. The local site can decide how to report the time – as percentage of effort, hours per week,
calendar-months, etc.
3. One potential model, that provides a great deal of clarity, is to report the effort available for
research as a percentage of the time at the affiliate, a percentage of time at the VAMC, and then
as a proportion of the total professional effort between the two sites combined. (See example
below.)
4. It is suggested that the MOU not include specific salary figures as this causes it to appear as a
contract that is promising a specific level of compensation. This is also problematic as salaries
change at irregular intervals and might require updating the MOU.
5. The
final signatory on behalf of the VA should be an individual who does not have a disqualifying
financial interest in the affiliate.
a. Investigators who plan to earn University salary under research grants that will fall under
the MOU should not be VA signatories to the MOU.
b. Additionally, while the VA official may have an in-name-only faculty appointment at the
affiliate, the VA official should not be compensated by the affiliate.
c. Disqualifying compensation includes current and ongoing benefits of significant monetary
value, including but not limited to wages, salary and other taxable benefits such as affiliate
contributions to life insurance, disability insurance, retirement plans, and subsidized tuition
benefits for employee or family members.
d. Benefits that are not considered disqualifying compensation include:
i. General faculty benefits that are given to all faculty members by virtue of their
appointment and that are not part of the individual’s particular employment
arrangement. These are usually of minimal value or are required by the faculty

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 109 of 149
appointment, such as parking permits, library access, admissions to artistic and
athletic events, and access to online university resources, office space, and the like.
ii. Royalties and other payments earned from patents or copyrights.
iii. The use of titles and honorifics associated with faculty membership.
iv. Benefits to which an employee had previously accrued entitlement during prior
employment with the affiliated institution, such as funds within a retirement
account. A benefit was previously accrued if its receipt is not contingent upon
continued current association with the affiliate.
v. In addition, malpractice coverage for uncompensated clinical care duties is not
considered disqualifying compensation for a clinician.
6. Local sites may choose to have the ACOS/R&D and/or the Service Chief also sign off on the MOU
even though they have dual appointments and receive salary from the affiliate. Their signature
on the MOU would reflect that they are aware of the terms of the MOU and would be clearly
distinguished from the signatures of the parties to the MOU. They would not be considered the
final signing authority on behalf of the VA and by their signatures would not be considered
participating personally and substantially in the MOU.
7. VA employees are reminded that they may not represent the University back to VA or any other
Federal agency on a matter in which the U.S. is a party or has an interest in the matter.
NIH Requirements for Time and Effort MOU
https://grants.nih.gov/grants/policy/nihgps/nihgps.pdf
Note that it is permissible to have a reported effort of greater than 40 hours per week between a VA
position and salary from an NIH grant at the affiliate. For example, an investigator could be full-time VA
(40 hours per week) and receive a salary for 10 hours per week from an NIH grant at the affiliate = 50
hours per week total. NIH does not set a maximum on the combined time, but states that it should be
“reasonable”. Most VAMCs have used 60 hours per week as the maximum.
(From NIH’s Grant Policy Statement, as of December 2021.)
Page IIB-123 17.3 VA-UNIVERSITY AFFILIATIONS
Investigators with joint appointments at a VAMC (VA hospital) and an affiliated university must have a
valid MOU that specifies (at both the university and the VAMC) the title of the investigator’s
appointment, distribution of compensation, the responsibilities of the proposed investigator, and the
percentage of effort available for research at each institution. The MOU must be signed by the
appropriate officials of the recipient and the VAMC and must be updated with each significant change of
the investigator’s responsibilities or distribution of effort and, without a significant change, not less than
annually. The joint VA/university appointment of the investigator constitutes 100 percent of his or her
total professional responsibilities. However, NIH will recognize such a joint appointment only when a
university and an affiliated VA hospital are the parties involved.
A grant application from a university may request the university’s share of an investigator’s salary in pro-
portion to the effort devoted to the research project. The institutional base salary as contained in the
individual’s university appointment determines the base for computing that request.
The signature of the AOR of the submitting university on an application to NIH that includes such an
arrangement certifies that the individual whose salary is included in the application serves under a joint
appointment documented in a formal MOU between the university and the VA, and there is no
possibility of dual compensation for the same work or of an actual or apparent conflict of interest.
Under the above-described arrangement, there is no involvement of a VA-affiliated non-profit research
corporation, which is eligible to apply for and receive NIH grants as a non-profit organization. The

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 110 of 149
limitations on the payment of Federal salaries apply (see Allowable and Unallowable Costs in this
chapter).
Page IIB-126
A part-time VA employee at VANPCs for which NIH grant funds are used to pay the
differential between the individual’s VA part-time salary and the salary level for a full-
time VANPC commitment in proportion to the level of effort devoted to the project.
Compensation must be in accordance with the established policies and salary structure
of the VANPC and the total number of VA and VANPC hours should not exceed a full-time
position. Therefore, if the PD/PI has a part-time appointment with the VANPC, an
appropriate portion of the individual’s salary that would otherwise be supported by the
non-profit VANPC may be charged to the NIH grant. The work paid for by the VANPC
must not be for the same project paid for by VA time for VA salary in accordance with
the VA policy set forth in the VHA Handbook 1200.17.
Examples of templates (suggestions only, not required)
Total Affiliate U. (must = 100%) 100%
VAMC
Staff physician
Responsibilities % of affiliate appt Proportion of Total Professional Effort
Research 25%
Other 75%
Total Affiliate U. (must = 100%) 100%
Total Affiliate University + VAMC (must total 100%)
John Smith MD
Affiliate University
Title: Associate Professor Dept: Medicine
Responsibilities % of affiliate appt Proportion of Total Professional Effort
Research 50% 16.66%
Other
50%
16.66%

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 111 of 149
SECTION 16 - VA Non-Profit Corporations
Besides academic affiliations, VA medical centers with research programs also may affiliate with
separate nonprofit organizations.
“Formed in 1992, the National Association of Veterans’ Research and Education Foundations (NAVREF)
is the 501(c)(3) nonprofit membership organization of research and education foundations affiliated
with the Department of Veterans Affairs medical centers. These nonprofits, also known as VA-affiliated
nonprofit research and education corporations (NPCs) are authorized by Congress under 38 USC
Sections 7361-7366 to provide flexible funding mechanisms for the conduct of research and education
at VA facilities nationwide.”
15
There are 84-member VA nonprofits.
Every medical center should have an MOU documenting the facility’s relationship with the local research
nonprofit organization.
Let’s back up a second. When an organization is a nonprofit, does that mean it’s not allowed to make a
profit? Of course, not!!! Another term for a 501(c)(3) organization is “public benefit organization.” This
term better describes why a nonprofit organization is a tax exempt. Nonprofit or public benefit
organizations are distinguished from for-profit organizations by the following:
1. They don’t pay taxes on their business-related excess income over expenses (or “profit)
a. They a
re liable to pay taxes on income earned from Unrelated business net income, by
the way.
2. They a
re not OWNED by anyone. (No shares of stock.)
3. They are governed by a board of directors who is responsible for protecting and ensuring the
public benefit mission.
4. They can accept donations as income that has special tax implications to the donor.
a. B
ut if you pay a nonprofit for a good or a service (e.g. class, daycare, Christmas tree,
etc.), that is not a donation.
With that in mind, nonprofits can behave very similarly to for-profit organizations. Executives can make
(huge) salaries, employees are under the protection of the same labor laws as everyone else, and the
corporation can produce goods and/or services for sale.
OK. Back to the VHA. Here are some examples of names of VA non-profits:
• Biomedical Research Institute of New Mexico (Albuquerque VA)
• Institute for Medical Research (Durham VA)
• Research! Mississippi (Jackson VA)
• Southern California Institute for Research and Education (SCIRE) (Long Beach VA)
• Louisiana Veterans Research and Education Corp. (New Orleans VA)
As you can see, the names vary quite a bit and do not always indicate the relationship to the VA.
Also, be advised: having this “alternate” funding mechanism for research and education may often
baffle and confuse others in the VA. Reactions can vary from, “You’ve got all the money you need from
the non-profit,” to “That’s got to be illegal.”
15
NAVREF webpage: http://www.navref.wildapricot.org/page-18055

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 112 of 149
Usually, the VA nonprofit handles the contracting, purchasing, and HR activities pertaining to studies
funded by:
• NIH
• DoD
• Pharmaceutical Companies
• Contract Research Organizations (these are companies hired by pharmaceutical companies to
run their multisite studies (e.g., Quintiles, Covance)
• Associations and Societies (e.g., American Cancer Society, Muscular Dystrophy Association)
o These organizations are notorious for disallowing overhead (indirect costs).
Try putting some indirect costs as direct costs in the budget.
These could be human, animal, or basic science studies.
Most of the non-profit’s expenses are going to go for salaries, travel, equipment, and supplies.
When the VA NPCs accept funding from pharmaceutical companies, contract research organizations on
behalf of pharmaceutical companies, or biotechnology companies, this type of funding is generally via a
contract (i.e., there is a scope of work that is agreed upon that will be done by the VA NPC/local
investigator that is requested by the company). Thus, a Cooperative Research and Development
Agreement (CRADA) will be necessary. Prior to the development of a CRADA, discussion between the
Investigator and the company may occur and Confidential Disclosure Agreements are usually agreed
upon initially to protect discussion of proprietary information. Templates of Confidential Disclosure
Agreements are available on the VA Technology Transfer webpage for Forms, Templates, and Model
Agreements. The ACOS/R&D can usually sign off on Confidentiality Agreements on behalf of the VA
Medical Center. The CRADA is reviewed and vetted by the VA Office of General Council (OGC) and the
signatory for the VA is the Medical Center Director. The CRADA spells rights to intellectual property
should any emanate from discoveries made during the research. CRADA templates for Clinical Trials,
Principal Investigator Initiated, and Investigational Device Clinical Trials are available on the VA
Technology Transfer webpage for Forms, Temp
lates, and Model Agreements. The use of Master CRADAs
(CRADA templates that are pre-approved by the pharmaceutical company and the VA) has improved the
process flow but not all the pharmaceutical companies have Master CRADAs with the VA.
One of the major items in the CRADA that needs serious thought is budget development for the scope of
work that needs to be done. While there is no one set template regarding budget development, the
Investigator and VA NPC must consider the following: personnel costs (e.g., that of a study coordinator
and to be sure that a study coordinator who may be involved with more than one clinical trial may not
only be involved in direct research patient interaction but also devotes time to much of the regulatory
and compliance paperwork for study review by VA research committees but time to interact with study
monitors from the pharmaceutical company or contract research organization, or other regulatory
visitors), study patient costs which may entail reimbursement for time commitment to the research
project and travel costs; Institutional Review Board fees or other fees necessary to help support the
human subjects protection program overall; record storage costs (e.g., consider off-site storage costs at
approved VA off-site storage sites).
Generally, study and comparator medications or devices are provided by the pharmaceutical or
biotechnology company, but the Investigator and VA NPC needs to be sure that if the pharmaceutical or
biotechnology company expects the VA to pay for the comparator medications or appliances, that
Pharmacy and/or Prosthetics may need to be consulted as to costs since these costs may need to be
reimbursed to the VA Medical Center. Finally, should the study require that other Medical Care services
be needed to perform the research (e.g., imaging studies, laboratory testing), that allowances in the
budget be made to reimburse Medical Care appropriation when these services are needed exclusively
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 113 of 149
for the research project or in addition to routine clinical care as required by the research project.
Mechanisms for the VA to bill the VA NPC can be worked out to reimburse Medical Care appropriation,
as needed.
Also, while many private foundations that fund research projects may have a limit on indirect costs that
are provided or some not allowing any indirect costs, many VA NPCs will administer these types of
grants as they are usually aimed at young or beginning investigators. Many VA NPCs understand that a
young or new VA investigator needs a start to his or her research career and getting that first small grant
is helpful to generate more data for larger grant submissions to federal agencies that do provide indirect
costs and to get an initial publication out.
All IT equipment proposed to be purchased through the non-profit organization should be vetted by the
local IT department. It would be helpful to be sure that IT equipment purchased by the VA NPCs would
be compatible to accept the VA image if thought is to have that IT equipment enabled on the VA
network. In most cases, IT equipment purchased by the VA NPC would need to be donated to the VA so
it can also be put into the VA inventory management system as required. In many cases the VA NPCs
can supply the equipment. Remember – Data for approved VA research is VA data. The ISSO and Privacy
Officer have responsibilities to protect it.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 114 of 149
SECTION 17 – Local Facility
In some VA locations research is very up front and present at the VA facility (often due to a strong
research-oriented university affiliation) but for smaller to medium sized research programs, research
may be overlooked, or even ignored in favor of patient care. You may have to figure out ways to bring
research to the forefront of the local facility leadership. You may find that there are many
misconceptions about research that go back a long way. Some things you may hear (or, I have heard):
• Research is not about patient care
• Research is illegal
• Research is making our Veterans into guinea pigs
• Nurses shouldn’t help with research studies
• Research is “special.” Doesn’t have to play by the same rules.
• Researchers don’t work as hard as we do; they’re never here.
Bottom line: Research can be mysterious at best at some locations and disdained at worst at others.
It’s a good idea to participate in facility committee activities. A representative from research on the
facility space committee, the Environment of Care Committee, or even the Radiations Safety Committee
(research should already be on this latter committee) is a good idea; this helps keep research informed
about who is doing what at the local facility and can help research maintain or even expand its space
needs. Frequent and positive interactions with facility services help develop support for research. Look
for opportunities to help and inform. The ACOS/R&D is usually a member of the Medical Executive
Committee or other equivalent composed of clinical Service Chiefs and chaired by the Chief of Staff. The
ACOS/R&D can inform clinical leadership of events, accomplishments, and situations in Research
needing the attention of this group.
Services You’ll Interact With:
• Fiscal
o Even though research money is not doled out through the network (like facility money),
fiscal is notified of funds received and responsible for transferring it into the CP’s
determined by the research service.
o You or your budget analyst will have frequent contact with someone in fiscal
responsible for managing research funds.
o Do you make patient payments on VA studies? You may have to go through fiscal and
the facility agent cashier to make this happen.
• HR
o All
hiring must go through HR
16
Position descriptions
Classification
Posting positions
Hiring
o IPAs
At some facilities, IPAs are required to be signed off by both HR and someone
from the network contracting office.
o Employee Relations
o Performance Awards
16
See Section 4: Human Resources for the updated process involving two DEU specialists and VA Subject Matter
Experts (SMEs) to help facilitate the recruitment of all ORD funded positions
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 115 of 149
• Engineering
There is a constant need to have things fixed, adjusted or assessed:
o Air conditioning or heating
o Leaks
o Power issues
o Broken sprinkler heads, ceiling tiles, door handles, etc.
• IT
Someone’s computer isn’t working or needs to be replaced. Printers break daily.
• Safety Office
With all the bio-hazardous materials used in research, your facility Safety Office may have a big
interest in the Research Service. Sometimes the Safety Office is unaware that research has several
handbooks dealing with research safety, so you might need to educate them about that. You will
have a lot of interaction with the facility Chemical Hygiene Officer and maybe the Radiation Safety
Officer.
• Housekeeping (Environmental Management Service)
Hopefully you don’t need to have much contact with housekeeping, but you might if you have a
housekeeper that is uncomfortable with or not knowledgeable about research.
You may have staff that need to be relocated. This may involve much assistance from the
housekeeping service.
• Executive
Since the Facility Director is the responsible and signatory official on many research documents, you
will often interact with the Executive office to get these documents completed.
o Your Director and/or Chief of Staff will be ex officio members of your R & D committee.
o The Associate Director (for Operations) may be involved helping you deal with
Engineering, Fiscal or Housekeeping services.
o Members of the Executive service will participate in inspection or survey entrance and
exit briefings.
Emergency Procedures
Your facility will have an Emergency SOP or system in place with detailed procedures and systems to
address an emergency. The AO and ACOS/R&D may be involved in facility emergency drills.
If you have an animal facility you will have a VMU Emergency plan since there are requirements for the
proper handling of animals in an emergency.
Your research program will also have an Emergency Plan of some sort that deals with how the research
labs respond in an emergency. This should be coordinated with and through your facility safety office
and be integrated in with the overall facility emergency plan.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 116 of 149
SECTION 18 – Safety & Security
Directives & Handbooks
The following directives and handbooks address research safety and security:
• VH Directive 1058.01 - Research Compliance Reporting Requirements
• VHA Directive 1200.08 – Safety of Personnel and Security of Laboratories Involved in VA
Research
• VA Directive 7700 – Occupational Safety and Health
• VA Handbook 7700.1 – Occupational Safety and Health Handbook
• VHA Directive 7701 – Occupational Safety and Health Program
• VHA Handbook 7701.1 – Occupational Safety and Health Program Procedures
• VA Handbook 6500 – Information Security Program
• VA Handbook 1200.12 – Use of Data and Data Repositories in VHA Research
SRS (Subcommittee on Research Safety)
The Research and Development Committee has a subcommittee to assess the safety requirements of
each research study (human, animal, and basic), usually referred to as the Subcommittee for Research
Safety (SRS). This committee must ensure that research is conducted safely in terms of the use of
chemicals, biological agents, reagents, procedures, exposure, protection, storage, and training. This
committee is not responsible for assessing the scientific merit or fundability of a protocol, although it
may make recommendations to the appropriate committee pertaining any of these concerns.
It is optimal if all subcommittees of the Research and Development committee (R&DC) can easily and
freely communicate with each other, as well as with the R&DC.
As AO, you will be an ex officio, non-voting member of the SRS. It is your role to ensure that the Safety
Committee has all the information it needs to make informed decisions and is performing its duties, as
described in your facility’s SOPs.
Safety Compliance
According to the VA Directives and Handbooks, there are several required safety inspections. These can
be combined with other facility inspections, if members of your safety committee belong to facility
inspection groups, e.g., environmental rounds.
Do you have a separate security committee, or is it part of another committee? Security involves not
only information and data, but also of premises or lab areas (physical security).
ORO frequently publishes checklists on their website that can be used to help you ensure that the
research service is covering all the bases for safety and security:
Security
Each research program needs to address the requirements as it pertains to security. This can mean
physical and personnel security, as well as data security.
Each year a multidisciplinary team (members include HR, Police, Safety, and Research) must review the
research facilities and program for research security.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 117 of 149
If a subcommittee is formed for this purpose it would report to the R&DC through the Safety committee,
or it might be a direct report to R&DC. Research Security can also be addressed as a work group or task
force and therefore would not require a separate SOP and program review.
Information System Security Officer (ISSO)
Also, mentioned previously in the IT section, the ISSO is the person at your facility that is responsible for
data security. The ISSO will review all initial submissions of research protocols to ensure that study data
is managed, according to VA regulations. The ISSO will resolve any questions or concerns directly with
the researcher submitting the study.
As you know from your Privacy and Information Security Training, any breaches of information security
are reported by the person involved directly to the ISSO, ACOS/R&D and the PO (if it involves people).
The ISSO may have to report the matter to the National Security Operations Center (NSOC). The ISSO
should notify the ACOS / R&D that an NSOC incident has been entered. This triggers the need for the
Director to notify ORO of the incident within 5 business days. See VA Handbook 1058.01 – Research
Compliance Reporting Requirements for more details. https://www.va.gov/ORO/oropubs.asp
The ISSO should be an ex officio non-voting member of either the IRB or the R&D Committees.
Police Service
Your facility has a Police Service that is the central monitoring group for security access systems at your
facility, including research areas. As AO, you are responsible for reviewing security access logs for
research weekly. You may need the Police Service to generate the reports for you unless they give you
limited access to generate them yourself.
Anytime there is an incident of theft or vandalism, etc., make sure it is reported to the Police Service by
the people involved or knowledgeable of the incident.
If you need access to the facility over the weekend, you may need to be given access by the police
service. As AO, you should not need any further approval for this but if your staff will be coming in on
the weekend, you may need to notify the Police Service and give them your approval.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 118 of 149
SECTION 19 – Privacy
Directives & Handbooks
• VA Directive 1605.01 – Privacy and Release of Information
• VA Directive 1605.02 – Minimum Necessary Standard for Protected Health Information
• VA Directive 1605.03 – Privacy Compliance Assurance Program and Privacy Compliance
Monitoring
• VA Handbook 6500 series
A good location for Privacy information is the VA Privacy Hub (VA Privacy Service Privacy Hub - Home
(sharepoint.com). You can find general information as well as a listing of privacy officers. As mentioned
above in the IT Section, every facility has a Privacy Officer (PO). You should address any questions you
have about Privacy to the PO. However, some POs have little or limited experience with the research
setting. They might need help with research Privacy issues. Your RCO may be helpful and there is also a
Privacy Board within ORD that can help resolve issues and answer questions.
The Privacy Officer needs to review each initial submission of human research studies. Privacy is not a
concern in animal studies. The PO will work with the research team to address or correct any privacy
issues.
The Privacy Officer should be an ex-officio non-voting member of either the IRB or the R&D committee.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 119 of 149
SECTION 20 – Animal Program
Handbooks
The handbook that covers the animal program within the VHA is:
• VHA Handbook 1200.07 - Use of Animals in Research which was reissued on Nov. 23, 2011.
The other document that is heavily used to ensure that animal research is performed ethically is Guide
for the Care and Use of Laboratory Animals (NRC 2011). The 8
th
edition (© 2011) can be found here.
Veterinary Medical Unit
The area of the medical center where the animals are kept is called the Veterinary Medical Unit (VMU).
This area is led by the veterinarian for the research program or Veterinary Medical Officer (VMO). The
VMO can either be a VA employee or a contractor and must be a licensed veterinarian.
The VMO directs the operations of the VMU, oversees the animal care staff and ensures compliance
with animal welfare laws, regulations and policies concerning VA animal research.
17
VMU staff are responsible for feeding, caring for, and maintaining a clean environment for the animals.
They keep a daily census of
animals and notify researchers when there are changes in the
condition of their animals. They monitor the temperature in the animal rooms. They may be
engaged in quarantine activities as well as the disposal of animal carcasses. They handle the
intake of new animals into the VMU. Sometimes VMU staff assist researchers with their
research activities, but this varies by facility and is not considered part of their normal duties.
Institutional Animal Care and Use Committee
The animal program is governed by an animal committee, usually referred to as the Institutional Animal
Care and Use Committee (IACUC), which is another subcommittee of the R&DC. This could be a local VA
IACUC or the IACUC of the academic affiliate.
The IACUC reviews animal protocols and ensures compliance with regulations governing the use of
animals in research studies. Senior research staff, including the AO, may serve on the IACUC as non-
voting members.
The AO should help the IACUC make informed decisions by being knowledgeable of the animal program
SOPs. Refer to your local SOP and/or Section 8 in Handbook 1200.07 for detailed information. The RCO
may be an invited guest of the IACUC; this can be a permanent invitation if included, as such, in the
IACUC SOP.
AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care)
AAALAC International is a private, non-profit, internationally accepted organization that accredits
institutions that meet its standards of humane treatment of animals in science. These standards go
beyond minimum legal requirements to promote excellence in animal care and use. Participation in
17
VHA Handbook 1200.07 – Section 6 b. (5)
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 120 of 149
AAALAC’s accreditation program is voluntary, but VA policy requires all VA animal research programs to
earn and maintain full accreditation.
Assurance
Your research program’s animal assurance (the counterpart to the human research Federal-wide
assurance) is managed through the Office of Laboratory Animal Welfare (OLAW) which is a branch
within the National Institutes of Health (NIH).
Reports
Every year the Research program needs to provide information to different entities about its animal
research activities.
• USDA Annual Report – Due on November 15
• AAALAC
• Annual reports
• Triennial Program Description
• ORD Annual Station Data Report – due January 15
• OLAW Annual Report – due on January 31
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 121 of 149
SECTION 21 – Site Visit Coordination
The research program is often inspected, surveyed, and site visited by internal and external groups.
These can be “for cause” (an allegation of non-compliance has been reported or noted) or routine.
Some entities that may review the research program are:
• ORO
• OIG
• GAO
• FDA
• USDA
• OSHA
• CDC
• ITOC
• AAHRPP, or other human studies accreditation group
• AAALAC
• VISN contractors to review:
o Safety
o Security
o Others
• EPA
You will probably have facility groups that want to perform inspections or walk-throughs:
• Annual Workplace Evaluation (AWE)
• Environment of CareRounds
• Safety
• ISSO
• IT or IRM
• Controlled Substance
• Space
Hardly a week will go by during which you are not coordinating, or participating, in some type of
research program review. You may need to help coordinate the submission of documents for the
review. These would include minutes, agendas, SOPs, and reports.
You may need to help set the agenda for the inspection and coordinate the people needed to be in
attendance.
In some instances, you will be asked to help with hotel reservations, meals/restaurant locations, and
transportation for the visiting inspection staff.
Remember, you are not alone!
There are many other people who are responsible for the success and compliance of the research
program. As the AO, you have an enormous role in this – but, the ACOS/R&D, RCO, and committees are
responsible entities, as well.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 122 of 149
SECTION 22 – IBC Registration and Annual Reporting
Institutional Biosafety Committee
If your research program is engaged in research involving recombinant DNA then you will need to have
committee oversight from an Institutional Biosafety Committee (IBC) which is registered with the Office
of Biotechnology Affairs (OBA) at the NIH.
Some facilities have their safety committee function as the IBC. The IBC will need to have and follow an
SOP for how to function and document its activities. You may use the affiliate’s IBC if your MOU with the
affiliate university allows for it.
The research program will need to name a Biological Safety Officer (BSO), who will be a member of the
IBC. The BSO will also be responsible for performing periodic inspections of areas involving rDNA
research to ensure that lab standards are rigorously followed.
Who Must Be On the IBC
• No fewer than five members, so selected that they collectively have experience and expertise in
recombinant DNA technology and the capability to assess the safety of recombinant DNA
research, and to identify any potential risk to public health or the environment.
• At least two members shall not be affiliated with the institution (apart from their membership
on the IBC), and shall represent the interest of the surrounding community, with respect to
health and protection of the environment.
• At least one individual with expertise in plant, plant pathogen, or plant pest containment
principles when experimentation uses plans (probably not applicable in the VA setting).
• The IBC shall include at least one scientist with expertise in animal containment principles when
experiments using animals are done.
• When the institution conducts recombinant DNA research at BL3, BL4, or Large Scale (greater
than 10 liters), a BSO is mandatory, and shall be a member of the IBC.
• When the institution participates in or sponsors recombinant DNA research involving human
participants, the institution must ensure that the IBC has adequate expertise and training (using
ad hoc consultants as deemed necessary).
• A member representing the laboratory technical staff.
The Institution shall file an annual report with NIH/OBA, which includes:
1. A roster of all IBC members clearly indicating the Chair, contact person, Biological Safety Officer
(if applicable), plant expert (if applicable), animal expert (if applicable), human gene therapy
expertise or ad hoc consultant (if applicable), and
2. Biographical sketches of all IBC members (including community members).
3. If utilizing the affiliate IBC, you must include your MOU.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 123 of 149
SECTION 23 – Human Research Protection Program
The Human Research Protection Program (HRPP) encompasses the IRB and all matters pertaining to
research involving human subjects at the VA facility. It is what was assessed by AAHRPP or other human
research protections program accreditation organizations when considering accreditation of your
facility.
The HRPP is comprised of facility senior management, members of the research office, the IRB, the
R&DC, and all the members of the research team conducting human studies. Together, this group is
responsible for protecting the rights, safety, and well-being of subjects enrolled in research projects.
The definition of HRPP found in VHA Directive 1200.05 is: “A comprehensive system to ensure the
protection of human subjects participating in research. The HRPP consists of a variety of individuals and
committees such as: the VA facility Director, Associate Chief of Staff (ACOS) for Research and
Development (R&D), the R&D Administrative Officer, the R&D Committee, the Institutional Review
Board (IRB), other committees or subcommittees addressing human subjects protection (e.g., Biosafety,
Radiation Safety, Radioactive Drug Research, Conflict of Interest), investigators, IRB staff, research staff,
health and safety staff (e.g., Biosafety Officer, Radiation Safety Officer), compliance officers, information
security officers, privacy officers, and research pharmacy staff. The objective of this system is to assist
the institution in meeting ethical principles and regulatory requirements for the protection of human
subjects in research.”
Your research program will have at least one Standard Operating Procedure (SOP) dealing with the HRPP
– or maybe several. It is regulated by VHA Directive 1200.05.
Office of Research Protections, Policy and Education
At ORD, there is an office called ORPP&E (the Office of Research Protections, Policy, and Education) that
provides resources to support the implementation and management of the human research protection
program. Their website is located here: https://www.research.va.gov/programs/orppe/default.cfm .
ORPP&E is responsible for 1) developing policy and guidance on VA human research protection; 2)
training and education for human research protection; 3) ensuring that all VA facilities that conduct
human research achieve and maintain full accreditation of their HRPPs; and 4) creating and
implementing the VA Central IRB. (NOTE: ORO, not ORPP&E, is responsible for FWAs.)
FWA or Federal-Wide Assurance
Because your HRPP is part of a federal organization it must have a Federal-Wide Assurance (FWA). The
HRPP operates under this FWA. Each FWA must be renewed every 5 years or within 30 days when there
is a change in the facilities Institutional Official, who is the signatory official on the assurance. Each time
the FWA is updated, a VA Addendum must also be submitted through the VHA Office of Research
Oversight (ORO) to OHRP. (Note: there is OHRP assurance training that must be completed by the
signatory officials.)
VHA Handbook 1058.03, published by ORO, deals with issues related to assurances.
Belmont Report
This report was issued by the National Commission for the Protection of Human Subjects of Biomedical
and Behavioral Research on September 30, 1978, and was later published in the Federal Register on
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 124 of 149
April 18, 1979. It took its name from the Belmont Conference Center, located in Elkridge, MD, where the
document was drafted in part. The three fundamental ethical principles are:
1. Respect for persons: Protecting the autonomy of all people and treating them with courtesy
and respect and allowing for informed consent. Research must be truthful and conduct no
deception;
2. Beneficence: The philosophy of “Do No Harm” while maximizing benefits for the research
project and minimizing risks to the research subject; and
3. Justice: Ensuring reasonable, non-exploitative, and well-considered procedures are
administered fairly – the fair distribution of costs and benefits to potential research
participants – and equally.
As part of your FWA, your institutional official will attest that your HRPP follows the principles of the
Belmont Report.
The Common Rule
Seventeen federal agencies (including the VA) joined Health and Human Services (HHS) in adopting a set
of rules for the protection of human subjects known as “the Common Rule.” These requirements are set
forth in Subpart A of 45 Code of Federal Regulations (CFR) Part 46 for the Department of Health and
Human Services (HHS). VA adopted the Common Rule as 38 CFR Part 16 which is identical to 45 CFR Part
46. Subparts B, C, and D were added to provide additional protections for pregnant women, human
fetuses, and neonates (B), prisoners (C), and children (D). VA has not formally adopted Subparts B, C,
and D, but some of their provisions are included in VHA Directive 1200.05.
On January 19, 2017, a major revision to the Federal Policy for the Protection of Human Subjects
was published and subsequently revised January 22, 2018 and again June 19, 2018 that requires
compliance of studies approved by the IRB or determined to be exempt by IRB on or after January
21, 2019. The revised Common Rule allows for continued compliance with the pre-2018 Common
Rule for those studies approved by the IRB or determined to be exempt prior to January 21, 2019.
Studies originally subject to the pre-2018 Common Rule may transition to the revised Common Rule
on or after January 21, 2019. If a study originally subject to the pre-2018 requirements is
determined to transition to the revised Common Rule the IRB will document and date the
determination within the minutes or within the electronic protocol file. Studies that transition to the
revised Common Rule must comply with all applicable 2018 Common Rule requirements on the
documented date.
Working with Boards and Committees
Research and Development Committee
Governed by VHA Directive 1200.01, the committee is tasked with oversight of the local research
program, broad areas of program development, risk management, and quality and performance
activities. Perhaps the most variability exists in how VA R&D Committees function, as they are no longer
tasked with individual protocol review. Some R&D Committees have retained this responsibility, and
some have not. The ACOS serves as the Executive Secretary of the R&DC and the AO may be an ex
officio, non-voting member of this committee and will be depended on to help the committee make
informed decisions. The SOP for the R&D Committee must reflect the procedures of your specific
processes.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 125 of 149
The complexity and intricate review processes for research studies through the R&DC and its
subcommittees cannot be overemphasized. It requires expert support, well-organized processes, and
explicitly written procedures. Resources: https://www.va.gov/ORO/orochecklists.asp
Institutional Review Board (IRB)
A significant component of the HRPP is the Institutional Review Board (IRB). The IRB must review each
human subject project, initially, and then at least annually after it has approved the project. The
elements of its review are numerous and multifaceted and predominantly guided by the Code of Federal
Regulations or the Common Rule and VHA Directive 1200.05 which deals with protection of the human
subject and the informed consent process. The IRB is a subcommittee of the R&D Committee.
The ACOS/AO may be ex officio, non-voting member of this subcommittee and may serve as consultants
regarding research processes. This means understanding program SOPs, in light of the issue being
discussed. The ACOS/AO should be sensitive to any potential, actual, apparent, or perceived conflicts of
interest and appropriately manage such conflicts. One solution is to limit the attendance of the
AO/ACOS to the general business portion of the meeting. This enables the ACOS/AO to remain aware of
how the committee is functioning but eliminates the perception that they are influencing the discussion
and review of individual protocols.
The IRB may meet monthly or more often depending on local
need (i.e., the size of the research program; number of protocols). A facility may have more
than one IRB, may use the VA Central IRB, the IRB of the academic affiliate, an IRB of another
VA facility, or an IRB of another federal agency. Requirements for using services of another
entity’s IRB are outlined in VHA Directive 1200.05.
Affiliate IRB
A Memorandum of Understanding (MOU) or Authorization Agreement with other VA facilities or
external organization(s) providing IRB services (see VHA Handbook 1058.03 and MOU Checklist:
https://www.va.gov/ORO/orochecklists.asp must be established and a written SOP should be in place
and must be consistent with VHA Directive 1200.05 when reviewing VA research. It should also cover
how communication will occur between the VA and the affiliate IRB (many times, this is the “weak link”
when problems occur in minutes from the affiliate IRB, and other communications regarding the VA
protocols that do not get back to the VA) and a sample template for the MOU for the VA-Affiliate IRB
arrangement. This information can be found on the ORO website (see the link provided above). You
must also ensure that the external IRBs of Record used by the VA facility hold current IRB registrations
with FDA/OHRP and provide updates to membership as required by VHA Handbook 1058.03.
Establishing the affiliate IRB as the IRB of record also needs CRADO (Chief Research and Development
Officer) approval if this is a change in their IRB of Record or establishes a new HRPP.
VA Central IRB
At VHA Central Office in the Office of Research and Development (ORD), there is a VA Central IRB. The
VA Central IRB reviews studies with greater than three sites where the study procedures are the same
across sites. Therefore, if you have CSP, RR&D, or HSR&D/QuERI multisite studies then you may use the
VA Central IRB as one of your designated IRBs.
While each VA facility has ultimate responsibility for its HRPP, the VA Central IRB provides expert
ethical and scientific review of VA funded multisite projects. The centralized review is meant to enhance
the efficiency of IRB review for these projects.
Each VA facility that plans to use the VA Central IRB must add the VA Central IRB to its FederalWide
Assurance (FWA) (in coordination with ORO per VHA Handbook 1058.03) and should have an MOU
between the VAMC, the local NPC, and the VHA Central Office describing the respective roles and
responsibilities of VHA Central Office, your local NPC, the local VA facility, and the VA Central IRB MOU
template. If one is not in place and your site has been identified as a potential participant in an ORD

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 126 of 149
funded multisite study, contact the VA Central IRB Administrator. Contact information is available
through the ORPP&E and VA Central IRB links on the ORD Website. (NOTE: The Institutional Official for
the VA Central IRB is the Principal Deputy under Secretary for Health.) There is variability among
research programs in regard to internal processes that encompass the review of VA Central IRB studies.
You should have internal SOPs that cover the review process for studies managed by the VA Central IRB.
VA Central IRB Liaison
There is a local individual designated by the Facility Director who is responsible for reviewing the VA
Central IRB determination and providing comments (e.g., on special local considerations and
requirements) before the final VA Central IRB approval. This person can be the local IRB chairperson,
ACOS, R&D chairperson, HRPP Administrator, or another qualified individual. All communication with
local site research personnel is through the Local Site Liaison who will be copied when approved study
documents are released to the Local Site Investigator or when an action is taken by the VA Central IRB
(such as, determining that a reported event is not related or not serious). The VA Central IRB has many
other communications with the local study teams in which the local site liaison is not involved. The
liaison is only copied once a final approval or determination is made. For reportable events that are
serious and further reportable, the VA Central IRB will also include other local site research and other
personnel on those communications per VHA Handbook 1058.01. For additional information, the Central
IRB website can be accessed at: www.research.va.gov/vacentralirb
IRB of Another Federal Agency
VHA Directive 1200.05 allows the use of an IRB of another Federal Agency through an Authorizing
Agreement. To date, the NCI Central IRB is the only other agency set up to work with VA, although there
is a push by NIH for use of single IRBs so there may be others, in the future. To use the NCI Central IRB,
the VA must develop SOPs to describe the roles and responsibilities of the VA and the NCI Central IRB –
these must be approved by ORO. Once the SOPs are approved and registration is done through NCI, the
VA must sign the Authorizing Agreement with NCI and amend its FWA to add NCI Central IRB as an IRB
of record. For more information, refer to the following National Cancer Institute (NCI) IRB Information
(va.gov)
Single IRB Mandate (https://www.research.va.gov/programs/orppe/single_irb.cfm)
January 20, 2020 was the compliance date of the cooperative research provisions in 38 CFR§16.114 of
the 2018 Requirements. As of January 20, 2020, all VA Non-Exempt Human Subjects Research approved
or transitioned to follow the 2018 Requirements of the Common Rule must use a single IRB unless an
exception applies to the research activity if more than one institution is engaged in the research and any
of the following applies to the multi-site research:
•
The cooperative non-exempt human subjects research is funded or supported by any of the
federal agencies or departments who agreed to apply 2018 Requirements.
•
Any of the other institutions are federal agencies or departments who agreed to follow the 2018
Requirements, including other VA Facilities
Subcommittee on Research Safety
Subcommittee on Research Safety (SRS) is covered elsewhere in the manual but should be considered as
part of the intricate and multifaceted review process described in this chapter.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 127 of 149
Non-Research Committees Responsible for the Review of Research
Pharmacy and Therapeutics Committee
In its review of research, the Pharmacy and Therapeutics (P&T) Committee is guided by the
VHA Handbook 1108.04. The P&T Committee must review research a research protocol when it
concerns a study that involves an investigational product, as well as any Cooperative Research
Agreement. This review must take place before the ACOS/R&D provides final notice of study approval or
initiation. It is suggested that there is research program representation on the P&T Committee and/or
the study PI attends the meeting in which their study is to be reviewed. It is also recommended that the
Research Administration/HRPP Office provide a template for P&T Committee Review that allows for
documentation and determination of study review. Depending upon the Research Program Office, it is
either up to the Administrative Office, or the study personnel to ensure that study material is submitted
for review by this committee within the accepted timeframe.
Radiation Safety Committee
In its review of research, the Radiation Safety Committee is guided by VHA Directive 1105 and VHA
Directive 1129. Per the directive, the Radiation Safety Committee must “review research protocols that
require the use of ionizing radiation as part of the research” and/or “involve the medical use of machine
sources of ionizing radiation.” This review must take place before the ACOS/R&D provides the final
notice of the study’s approval or initiation. It is suggested that there is research program representation
on the Radiation Safety Committee and/or the study PI attends the meeting in which their study is to be
reviewed. It is also recommended that the Research Administration/HRPP Office provides a template for
Radiation Safety Committee Review that allows for documentation and determination of the study
review. Depending upon the research program, it is either up to the Administrative Office or the study
personnel to ensure that study material is submitted for review by this committee within the accepted
timeframe.

Key Issues to Address When Terminating Human Subject Studies at a
VA Facility with a Human Research Protection Program
VHA Office of Research and Development, March 25, 2016
Category
Issue
Comment
1. Timeline
Define the date for the
projected closure of the
Human Research
Protection Program
(HRPP)
Intervals vary anywhere between 60 days to 1
year, with the usual range being between 3 to 9
months.
2. Notification of any
relying HRPPs
Any HRPP relying upon
the VA Facility’s IRB of
Record and/or Research
and Development
Committee must be
informed immediately as
this directly impacts the
relying VA Facility’s
HRPP.
3. Categorization of
studies:
(a) which studies are
non-human subjects,
exempt, or non-
exempt human
subjects
(b) funded (ORD,
other federal funding,
industry)
(c) collaborative
(d) Repositories – data
or biospecimen
Different studies will
have different issues
involving termination
and/or transition.
If there are any studies with CRADAs, the
termination clauses of those CRADAs need to be
reviewed.
For data and biorepositories, every effort should
be made to transfer those studies as permitted
within the approved protocol and the applicable
informed consent and HIPAA authorization.
4. Subject status:
Determine which
studies have subjects
which are currently
receiving any study-
related interactions,
interventions, and/or
follow-up monitoring.
Subject safety is the
priority for terminating
and/or transitioning any
human subjects study.
Determinations must be made for any
notifications that must be made for subjects in
any currently active human subjects studies.
5. Transfer of studies:
Determine if any of
the studies will or can
be transferred to
another institution
It is unknown whether
any of the studies will or
can be transferred to
other VA facilities.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 129 of 149
Category
Issue
Comment
6. Notification of
Investigators: Include
plan for terminating
and/or transitioning
studies as well as
actions they are
required to
implement, including
subject issues,
equipment inventory,
and records retention
for investigator study
records.
VA Investigators must be
informed quickly of plans
to terminate and/or
transition any studies so
that study-specific issues
can be identified.
7. Records issues:
Determine how
research records will
be made available for
any studies which will
be transferred, as well
as determine how
research records will
be retained for
purposes of VA’s
Record Control
Schedule.
8. Notification of key
parties
Depending upon the
study, additional
individuals or entities
(Collaborators, FDA,
funding agencies) may
need to be informed.
Those groups will be
identified based upon the
study.
Please note that the above is general guidance in brief bullet-format statements and is not intended to
address specific issues that may be associated with specific studies. ORD is available to assist as needed
on this topic.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 130 of 149
SECTION 24 – SOPs
SOPs are written based on local facility, central office, and ORO requirements, as well as on local laws.
They describe how the research program operates.
At many facilities, the AO writes the SOPs, but some SOPs might have some technical aspects to them
that are beyond the AOs training. In this case, others may need to be called in to help. At other facilities,
the committees are responsible for creating the SOP, and they may form ad hoc groups to complete the
task.
No matter who writes the SOPs for research, they will be carefully examined by ORO, and the
accrediting organizations and any deficiencies will be noted so that they can be updated and brought
into compliance. One bit of advice in writing SOPs is to not be so restrictive on elements of the SOP that
it would be difficult for the facility to keep compliant with the SOP.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 131 of 149
SECTION 25 – VA Technology Transfer Program (TTP)
Technology Transfer Program (TTP) facilitates the commercialization of invention to benefit Veterans
and the Public. Most often it involves licensing a patent to a company which will develop the invention
into a product that benefits the public.
• Inventions that use VA resources (funding, space, personnel, etc.) are disclosed to VA TTP.
• TTP, in conjunction with VA lawyers, sets a determination of rights (DOR) regarding whether the
VA asserts any rights to the invention. See Directive 1200.18
• A researcher’s academic affiliate may also assert rights to the invention and VA TTP works with
the affiliate’s technology transfer office to resolve these issues.
• Either the VA or the affiliate can take a lead in assessing the invention, filing a patent and
ultimately licensing the invention.
• Royalties are distributed to the inventor(s), the VAMC where the invention was made, and, if
applicable, to the affiliate.
• The VA researcher completes a certification form that identifies any VA resources used in the
invention. The ACOS/R&D reviews the form for accuracy and signs off on it.
Cooperative Research and Development Agreements (CRADAs)
TTP also reviews CRADAs in conjunction with the VA legal team dedicated to research issues (Specialty
Team Advising Research (STAR). A CRADA is an agreement between the VA and another party (typically
an industry partner) in which the partner typically provides funding and the VA provides personnel,
services, facilities, and other resources. The CRADA defines the responsibilities and obligations of each
party as well as their rights to intellectual property. CRADAs are typically executed between the VAMC,
the industry partner, and the VA-NPC (who administers the funds). In general, the CRADA will also spell
out intellectual property rights of each participating party a priori of any invention being made. See VHA
Directive 1206: Use of a Cooperative Research and Development Agreement (CRADA)
There are other guidance documents and templates for various other types of Intellectual Property type
issues such as Confidential Disclosure Agreements, and Material Transfer Agreements that can be found
at the Tech Transfer page at
https://www.research.va.gov/programs/tech_transfer/model_agreements/default.cfm
Since many of the researchers at the VA also have an appointment at the affiliate university, and are
thus dual employees, it is helpful to have the VA and the affiliate enter into an intellectual property
administration agreement. The older model is the Cooperative Technology Administration Agreement
(CTAA) but these will be transitioned to the newer Invention Management Agreement (IMA). Template
for the IMA can be found at the above link. These agreements cover which institution will pursue a
patent if warranted, which institution will seek marketing partners, and how royalties would be divided
between the institutions. Also, the VA Technology Transfer Office will usually accept the Invention
Disclosure Forms from the affiliate but still require the completion of the VA Certification form for each
VA inventor defined as a VA-paid employee, a WOC, or an individual on an IPA. The VA Invention
Disclosure and VA Certification Forms can also be found at the above link.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 132 of 149
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 133 of 149
SECTION 26 – Records Control Schedule
The Federal Records Act (FRA) requires that all Federal agencies make and preserve records that pertain
to the functions, decisions, and other actions of the agency. As applied to VA research records, the FRA
defines Federal records as all documentary material, regardless of physical form, or characteristics made
or received by a VA research program or, in accordance with, the transaction of the Agency’s business,
(i.e., the conduct of VA’s research programs and VA research) and that are preserved or are appropriate
for preservation as evidence of VA’s activities or because of their infor
mational value of data in them.
What are Records?
A record is created during the process of conducting business (e.g., correspondence, agreements,
studies, etc.); received for action (e.g., FOIA requests, controlled correspondence, grant applications,
etc.); documents VA activities and/or actions (e.g., calendars, meeting minutes, project reports, etc.);
mandated by statute or regulation (e.g., administrative records, dockets, etc.); supports financial
obligations or legal claims (e.g., contracts, litigation case files, IPAs, etc.).
A non-record is reference material (e.g., vendor catalogs, phone books, technical journals, etc.); a
convenience copy (e.g., duplicate copies of correspondence or directives, etc.); stock copy (e.g., VA
publications, etc.); draft or working copy (e.g., draft with no substantive comments, rough notes,
calculations, etc.). PLEASE NOTE: Some drafts are needed to support the decision trail or are required
by a records schedule.
Working Files include budget calculations, comments, rough notes, proposals, evaluations, preliminary
outlines for a report, lists of suggested topics to be included in a memorandum, informal comments
received on draft publications, and documents used to brief staff on a proposed item.
Personal Papers do not relate to, or have any effect upon, the conduct of agency business. Documents
created before entering government service, private materials brought into, created, or received in the
office that were not created or received in transaction of government business, and/or work-related
personal documents that are not used in the transaction of government business. PLEASE NOTE:
Personal planners and calendars might be records, if they document your activities for the VA.
The Life Cycle of Records
Creation: Records created, received, or collected through the daily transaction of business
Maintenance and Use: Filing, retrieving, use, duplication, printing, dissemination, release or exchange of
the information
Disposition: Assessment to determine the retention value of the record, in accordance with the record’s
schedule, and leads to either the preservation or destruction of the record
It could be a record if…
- It reflects significant actions taken during the process of conducting business.
- It contains unique, valuable information developed in preparing reports, studies, etc.
- It conveys unique, valuable information about your programs, policies, decisions, or essential
actions.
- It conveys statements of policy or the rationale for decisions or actions.
- It documents oral exchanges (in person or by telephone), during which policy is formulated or
related activities are planned or transacted.
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 134 of 149
- It adds to the proper understanding of the formulation or execution of actions or operations and
responsibilities.
- It documents important meetings and facilitates action by service or department officials and
their successors in office.
- It makes possible a proper scrutiny by auditing bodies external to the service or department.
- It protects the financial, legal, and other rights of the Government and of persons directly
affected by the Government's actions.
Examples of Records:
Meeting Minutes Functional Statements
Competency Folders Position Descriptions
Timekeeping documents Purchase Card transactions
Contracts Recruiting and Staffing files
Emergency Action Plans Tracking spreadsheets
Equipment Requests Committee Records
Employee Folders IPPS Invoices
Budget Planning Blueprints & diagrams
Travel Vouchers Maintenance Records
Getting Started
Records retention policies are in the Records Control Schedule (RCS) 10-1. FDA regulated studies have a
different records retention requirement and litigation holds will also alter the retention schedule of
certain records. Please see end of this section for links to resources.
1) Identify the Facility Records Manager
2) Locate all temporary storage locations within Research and Medical Center space where
research data/information are being stored.
3) Identify electronic storage locations of research data or information
a. W
ho controls access to these folders?
4) Create a spreadsheet with required information for tracking all hard copy and electronic
records, unless you have an electronic protocol management system that can track this
information for you. You will also want to check with your facility’s Records Manager to ensure
all required elements are being tracked.
At a bare minimum, one will want to ensure the following is being recorded:
a. Date of Inventory - Date initial inventory form was prepared, or date last updated
b. Person conducting the inventory - Name and telephone number
c. Record Title and Record Series - Give each series a title for brief reference or include the
generally accepted title
d. Record Description - A concise description of the records, which may include the
purpose, use, and subject content of the records
e. Records Control Authority - Records Control Schedule (RCS) 10-1, GRS, National Archives
f. Item # - Item No. specified in the applicable records schedule

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 135 of 149
g. Disposition - If the series has an approved disposition authority, list the schedule as
described on RCS 10-1, GRS, etc., i.e., Destroy after 1 year. If the series has no such
authority, list the files as “unscheduled,” and make sure they are preserved.
h. Location - Give the precise location of the series, File Cabinet #, etc.
If the series is located in more than one office, indicate multiple locations.
i. Arrangement - Indicate the arrangement of the records, i.e., alphabetic, numerical, etc.
j. Date Range - The earliest and most recent dates of the records in each series. These are
needed to schedule records, and to determine when to cut off, or break them and
transfer them to records centers or agency storage facilities.
k. Medium - Indicate whether the record medium is paper, CD/DVD, diskette, electronic,
audiovisual microform, maps/drawings, or a combination of these.
l. Cutoff - To cut off records means to break, or end, them at regular intervals to permit
their disposal or transfer in complete blocks to permit the establishment of new files.
Indicate how often the records are cut off and when the last cutoff occurred.
m. Reference activity - Rate the reference activity of a paper record series, after the regular
cutoff, by placing it in one of three categories:
i. Active (used more than once a month)
ii. Semi-active (used less than once a month)
iii. Inactive (not used for current operations)
n. Duplication - Indicate duplication in form or content. It can exist in the following ways:
i. Copies ma
y be in the same organizational unit or elsewhere in the agency. The
copies may contain significant differences or notations.
ii. Similar data or information may be available elsewhere in the agency either
physically duplicated or in summarized form.
o. Volume - The volume of records in inches/feet, where possible. When inventorying
audiovisual, microform, cartographic, and related records, provide an item count (e.g.,
1200 prints, 3500 negatives) where appropriate.
p. Legal Status - If the records qualify as vital records, specify whether they would be
needed in an emergency (emergency-operating records) and whether they are needed
to document legal or financial rights, or both. Also, indicate whether they are the
originals or duplicates.
q. Restrictions on access and use - Indicate any restrictions on access to, and use of, the
particular series. Such restrictions may result from statutes, executive orders, or agency
directives. Common types of restrictions are:
i. Privacy Act restrictions
ii. National security restrictions
iii. Freedom of Information Act restrictions
iv. Other applicable restrictions that may be specific to the agency
5) Create 7468
s
(http://vaww.va.gov/vaforms/Search_action.asp?FormNo=7468&tkey=&Action=Search) for all
records that are archived on-site as well as those off-site. This form is to track current records as
well as to certify destruction once records have reached their cutoff date.
6) U
pdate Initial Review and Continuing Review documents for all research projects to have PIs
disclose where they are storing their research data, both hard copy and electronic.
7) Submit the spreadsheet annually to the Facility Records Manager, or as requested
8) Submit weekly reports to the Facility Records Manager of all temporary storage locations of
records that are archived locally
9) Upon study closure or the departure of a principal investigator, ensure study closure forms
prompt the principal investigator to identify all their data locations and create a process to
transfer that data, hard copy and electronic, to a secure location for retention until the cutoff
date.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 136 of 149
Develop a local policy that describes the purpose, scope, definitions, responsibilities and procedures for
carrying out the records management program. Currently, off-site storage of VA records is approved at
NARA locations and is coordinated through the facility’s Records Manager. Payment for the storage of
records is also funded by the facility. For additional details regarding long term off-site storage, please
meet with your facility’s Records Manager, if your facility does not have a records manager, please
contact the VHA HIM Records Management Council by email at V[email protected]. T
hey can assist
in setting you up with a mentor and providing additional guidance on how to get a contract initiated for
long-term off-site storage.
Policies, Resources, and Helpful Links
FDA Records Retention
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.62
and
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=820.180
General Records Schedule
https://www.archives.gov/records-mgmt/grs.html
Guidance on ORD’s New Record Control Schedule
https://www.research.va.gov/resources/policies/guidance/rcs-guidance.pdf
Health Information Management website for records management
https://leaf.va.gov/VISN3/630/records_management/
Office of Management and Budget (OMB) Circular A-123
https://obamawhitehouse.archives.gov/omb/circulars_a123_rev
OMB Circular A-130 https://www.federalregister.gov/documents/2016/07/28/2016-17872/revision-of-omb-
circular-no-a-130-managing-information-as-a-strategic-resource
Title 44 U.S.C. Chapter 31
http://uscode.house.gov/view.xhtml?req=granuleid%3AUSC-prelim-title44-
chapter31&saved=%7CKHRpdGxlOjQ0IHNlY3Rpb246MzEwMSBlZGl0aW9uOnByZWxpbSkgT1IgKGdyYW5
1bGVpZDpVU0MtcHJlbGltLXRpdGxlNDQtc2VjdGlvbjMxMDEp%7CdHJlZXNvcnQ%3D%7C%7C0%7Cfalse%
7Cprelim&edition=prelim
Title 44 U.S.C Chapter 33
http://uscode.house.gov/view.xhtml?req=granuleid%3AUSC-prelim-title44-
chapter33&saved=%7CKHRpdGxlOjQ0IHNlY3Rpb246MzMwMSBlZGl0aW9uOnByZWxpbSkgT1IgKGdyYW51bGVpZDpVU0MtcHJl
bGltLXRpdGxlNDQtc2VjdGlvbjMzMDEp%7CdHJlZXNvcnQ%3D%7C%7C0%7Cfalse%7Cprelim&edition=prelim
Title 44 U.S.C. Chapter 35
http://uscode.house.gov/view.xhtml?req=granuleid%3AUSC-prelim-title44-
chapter35&saved=%7CKHRpdGxlOjQ0IHNlY3Rpb246MzUwMSBlZGl0aW9uOnByZWxpbSkgT1IgKGdyYW51bGVpZD
pVU0MtcHJlbGltLXRpdGxlNDQtc2VjdGlvbjM1MDEp%7CdHJlZXNvcnQ%3D%7C%7C0%7Cfalse%7Cprelim&edition=p
relim
Title 36 CFR Chapter XII, Sub Chapter B
https://ecfr.io/Title-36/CXIIsubchapB
Storage Standards Toolkit
https://www.archives.gov/records-mgmt/storage-standards-toolkit
Develop system for managing records for new, existing, and closed studies.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 137 of 149
VHA Directive 6300, Records Management
https://www.va.gov/vapubs/search_action.cfm?dType=1
VA Handbook 6300.01
https://www.va.gov/vapubs/search_action.cfm?dType=2
VA Handbook 6300.2.
https://www.va.gov/vapubs/search_action.cfm?dType=2
VA Handbook 6300.4.
https://www.va.gov/vapubs/search_action.cfm?dType=2
VA Handbook 6300.5.
https://www.va.gov/vapubs/search_action.cfm?dType=2
VA Handbook 6300.6.
https://www.va.gov/vapubs/search_action.cfm?dType=2
VA Handbook 6300.8.
https://www.va.gov/vapubs/search_action.cfm?dType=2
VA Handbook 0320.
https://www.va.gov/vapubs/search_action.cfm?dType=2
VHA RCS 10-1.
https://www.va.gov/vhapublications/rcs10/rcs10-1.pdf
VHA Directive 1907.01.
https://www.va.gov/vhapublications/publications.cfm?Pub=1

SECTION 27 – Financial Conflict of Interest
The FCOI Form
As a Federal agency, VA’s policies on FCOI must be consistent with requirements for government
employees of the Executive Branch published by the Office of Government Ethics (OGE) established by
the Ethics in Government Act of 1978. OGE is the agency providing overall direction, oversight, and
accountability of Executive Branch policies designed to prevent and resolve conflicts of interest.
Currently the FCOI disclosure form can be found on ORD’s Tech Transfer Program website (Research
Financial Conflict of Interest Statement located at
http://www.research.va.gov/programs/tech_transfer/model_agreements/default.cfm).
The FCOI Form needs to be completed by the Principal Investigator, Co-PI, Co-Investigators, or
Collaborators. The FCOI Form is project specific and is completed with a new project submission but also
must be re-done upon continuation renewal of the project.
Federal employees need to be mindful of 18 U.S. Code § 208 - Acts affecting a personal financial interest
“Except as permitted by subsection (b) hereof, whoever, being an officer or employee of the executive
branch of the United States Government […] participates personally and substantially as a Government
officer or employee, through decision, approval, disapproval, recommendation, the rendering of advice,
investigation, or otherwise, in a judicial or other proceeding, application, request for a ruling or other
determination, contract, claim, controversy, charge, accusation, arrest, or other particular matter in
which, to his knowledge, he, his spouse, minor child, general partner, organization in which he is serving
as officer, director, trustee, general partner or employee, or any person or organization with whom he is
negotiating or has any arrangement concerning prospective employment, has a financial interest”.
In general, researchers need to understand that the rules for Federal employee researchers are different
from the rules in academia. Federal employees are subject to criminal conflict of interest statutes as
well as the Standards of Conduct regulations. These rules, in simple terms, prohibit Federal employees
from participating in official VA matters (e.g., research) if the employee has a personal financial interest
or relationship that might affect their service to the Government are they acting in the best interest of
the public, Veterans and the Department or is there some element of benefitting themselves or
others. For the VA employee, the close relationship with the university affiliate only increases the
potential to have an outside interest that might disqualify someone from conducting research at
VA. One caveat – the mere existence of an outside interest does not automatically disqualify someone
from a certain study – the determination is fact-driven, and existence of an outside interest might mean
that a consult with OGC Ethics Specialty Team (EST) is in order. OGC has tools to manage some interests
that would otherwise be disqualifying. [By the way, a double-blind study does not resolve these types of
financial or relationship-based conflicts of interest.]
Under VHA policy, VA investigators must have a VA appointment (with or without compensation) or be
detailed or appointed under the IPA. All VA investigators are therefore subject to the Government
ethics rules be they at VA under a full-time or part-time appointment, salaried or WOC or under an IPA.
Contractors may not serve as investigators but may participate in research at the VA if it is in the terms
on the contract.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 139 of 149
Interests and relationships that might signal a need to contact VA OGV Ethics Team include:
• an outside entity that is funding/sponsoring the study or that owns or has licensed rights to
inventions that are involved in or affected by the study (“affected” should be initially thought of
in a very broad sense) – if the researcher or his spouse or minor child:
o has a financial interest in the company through ownership, stock holdings (including
publicly traded companies), being general partner;
o has a fiduciary responsibility toward the company because of service as
officer/director/trustee (usually on the board of directors);
o is an employee, consultant, contractor, agent, or otherwise does business with the
outside entity (e.g., speaker’s bureau, member of scientific advisory board, consultant)
(or if the researcher has held such a position within the past year); or
o is negotiating or has an agreement for future employment with the entity
• inventions are involved in the study – either the study is further research into the invention or
will affect the value of the invention (e.g., make it more-or-less likely to be commercialized) and
the invention is:
o made by the researcher,
o owned by the researcher, or
o owned by someone or some entity other than VA (including if that entity is the
university affiliate) in which the researcher has a relationship.
The Federal Technology Transfer Act at section 3710a, subparagraph (b)(3)(C), of title 15 United States
Code, permits a Federal employee, under a Cooperative Research and Development Agreement
(CRADA), to participate, as part of official duty, in effort to commercialize an invention made by the
researcher while in the employment or service of the Government. This means that with supervisory
approval and after putting in place a CRADA, and if necessary a waiver of the criminal conflict of interest
statute (208 waiver through EST), a VA researcher who has made an invention may assist the outside
company that has licensed the invention in the commercialization effort by providing scientific and/or
technical expertise or serving on the scientific advisory board. However, such assistance does not
extend to activities related to the management of the outside company or to the promotion and/or
marketing of its products to the public, in general. OGC Ethics should be consulted to determine if a 208
waiver is necessary.
Additional information on VA ethics can be found at
https://vaww.ogc.vaco.portal.va.gov/law/ethics/SitePages/Home.aspx
For an ethics consultation, email:
• OGCNorthAtlanticEthic[email protected] for CT, DC, DE, MA, MD, ME, NC, NH, NJ, NY, PA, RI, VA, VT,
WV
• OGCSouthEastEthics@va.gov for AL, FL, GA, KY, Puerto Rico, SC, TN
• OGCMidwestEthics@va.gov for IA, IL, IN, KS, MI, MN, MO, NE, ND, OH, SD, WI
• OGCContinentalEthic[email protected] for AR, CO, LA, MS, MT, OK, TX, UT, WY
• OGCPacificEthics@va.gov for AK, AZ, CA, Guam, HI, ID, NM, NV, OR, Philippines, WA
VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 140 of 149
208 Waivers
It may be possible to obtain a 208 Waiver and authorization that allows the researcher to participate in
research from which they would otherwise be disqualified, absent such a waiver, because of their
financial interest in the matters. The researchers will submit the information below to their VA Ethics
official who will work with them on a waiver. If approved by the Federal Government Office of
Government Ethics, the waiver will be processed through the ACOS for Research and signed by the MCD.
A request for a 208 Waiver includes the following:
1. Your full VA title, whether you are part time or Full time VA.
2. What is your “Area of Research” at VA? Please define this as broadly as possible in a sentence
or two.
3. Please list all patents that you own or are listed as an inventor on. For each, please state:
a. Who owns the patent at issue – VA-solely or jointly-owned by VA and the University?
b. Patent number, if applicable
c. Date issued or any other relevant dates
d. any other information relevant to the patent(s)
4. State in layman’s terms: Your proposed study and how it will affect this IP.
5. Does your spouse work for the VA or a hold a university appointment? Please describe and if so,
do they have any interest in the IP at issue?
6. Is there an outside company that is interested in licensing the IP?
7. If so, do you, your spouse, or minor child, have any equity interest in the company, currently, or
in the future?
8. A
re you (your spouse, or a minor child) an employee of the company licensing the IP, do you
consult for the company, or have any other financial interest in the company?
SECTION 28 – Publishing VA Research
Checklist for publishing VA research (funded by VA or used VA resources)
• Much of this information is covered in VHA Handbook 1200.19 – Presentation of Research
Results. Requirements for authors
• Note that the ORD service funding the study may have additional requirements; contact the
specific service or review the ORD website for more information.
• Acknowledge VA support Acknowledge VA employment in the manuscript
o If the work was funded by VA, include this statement:
“This work was supported [or supported in part] by [type of award, e.g., Merit
Review, Career Development Award, Pilot Project) Award # [award/project
number, e.g., I01 RX000123] from the United States (U.S.) Department of
Veterans Affairs [as applicable, indicate Biomedical Laboratory Research and
Development Service; Clinical Sciences Research and Development Service
(mention the CSR&D Cooperative Studies Program if applicable); Rehabilitation
Research and Development Service; or Health Services Research and
Development Service].”
o If VA only provided resources (e.g., facilities or patients), include this statement:
“This material is the result of work supported with resources and the use of
facilities at the [name and location of VA medical facility].”
• Acknowledge VA employment in the manuscript

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 141 of 149
o Acknowledge employment of VA authors with VA title, name of VA medical facility, city,
and state.
o Academic affiliate appointments can also be listed, but if research was funded only by
VA, the VA affiliation should be listed first.
• Include DVA/US Government disclaimer in the manuscript
o Include this disclaimer: “The contents do not represent the views of the U.S.
Department of Veterans Affairs or the United States Government.”
• Include NCT number in the manuscript
o If your publication concerns a clinical trial or observational study that was registered on
clinicaltrials.gov, include the NCT number in the publication. This allows the
clinicaltrials.gov website to link your paper to the trial registration.
• Notify VHA Research Publications
o Alerting VA Research Communications about upcoming publications or presentations is
particularly important when the topic is newsworthy, and VA can develop some
productive media relations or when the topic is controversial, and the assistance of
Public Affairs is likely to be needed
o Publications can be reported on-line at http://vaww.pubtracker.research.va.gov
. In th
e
near future, publication notifications will transition to VAIRRS (IRBNet)
o
• De
posit manuscript in PubMed Central if the research was ORD-funded research
o For specific instructions, see: http://www.ncbi.nlm.nih.gov/pmc/
o Deposited manuscripts must be made available to the public in PubMed Central no later
than 12 months after their publication in a journal.
o Some journals have an arrangement by which they will deposit the paper in PubMed
Central automatically. Participating journals are listed here:
https://www.ncbi.nlm.nih.gov/pmc/journals/
o Unless you are sure that the journal is posting, the author must post it. The following
link to a flow chart on “How Papers Get Into PMC” includes helpful information to assist
authors in working out how their papers may get into PMC:
https://www.ncbi.nlm.nih.gov/pmc/about/submission-methods/
SECTION 29 – Communications
Working with the Public Affairs Office and ORD Communications
VA Research is probably the “best kept secret” in VA and probably in all Federal organizations. Research
is the one bright area that continues to allow VA to put its best foot forward. Each VA Medical Center
should have a Public Affairs Office that is tasked to be an interface between the VA, its constituents, the
community, and the lay media. In many instances, the “hot” scientific discoveries or newsworthy items
are first picked up by the university affiliate (if there is an affiliated school with the local VA). Many of
the up and coming discoveries that could have wide potential benefit in medicine are first reported at
large national and international scientific meetings. These newswires are funneled through the
university affiliate even if the investigator and investigative team making the discovery has a VA
affiliation. The VA is usually the last to be acknowledged for positive items but generally the first to be
singled out for untoward events.
VA Investigators should be aware of the VA Public Affairs Office and usually the specific contact in that
Public Affairs Office when it is anticipated that a newsworthy item will surface. If contact with the Public

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 142 of 149
Affairs Office is unknown to the Investigator, the Investigator should contact the Research Office, either
the Associate Chief of Staff, Research or the Administrative Officer / Health Science Officer to put
him/her in contact with the correct person in the Public Affairs Office. In many instances, the VA Public
Affairs Officer has a direct line to the university affiliate Public Affairs Office so that joint
announcements or releases can be made giving credit to both the VA and the affiliate for dual
appointees and their discoveries.
Why is it important to be sure that VA receives credit for discoveries that emanate from VA research?
Positive news coverage and acknowledgement of VA science will continue to be a positive tool to ensure
that those having control over the VA research budget will have data to continue to support VA
research. VA constituents who read or hear about these discoveries and medical advances made by VA
Investigators will also be welcomed lobbyists and positive voices for VA research (these include VA
patients and Veterans Service Organizations). In order to assist the Office of Research and Development,
Veterans Health Administration to be aware of publications, presentations, interviews of VA
investigators, or other newsworthy events, this information can be entered into the VA ORD SharePoint
Pubtracker site: http://vaww.pubtracker.research.va.gov
.
Interestingly, despite having an annual Research Week celebrations, many employees of an individual
medical center do not know the existence of VA research or may know very little of the discoveries
ongoing at their medical center. Thus, some Public Affairs Office’s put out periodical communications
within the medical center highlighting VA research so that all employees can be engaged in the positive
features of a research program.
On many occasions, the VA Public Affairs Office may be involved in helping to curb negative VA research
image (especially with animal rights groups) by presenting the facts about how VA research has
contributed to advancing medical knowledge and contributing to diagnostics and cures that benefit not
only VA patients, but all patients afflicted with common diseases that are also experienced by VA
patients. Public Affairs Office usually reports directly to the Medical Center Director. Having an open
line of communication between the Research Office and the Public Affairs Office allows for the Director
to be aware of positive aspects of his or her Medical Center, but also gives the Medical Center Director a
head’s up for “prickly” items involving Research.
SECTION 30 – Research Misconduct
Transgressions in Research
Research Misconduct is narrowly defined as transgressions in one of the following three categories:
falsification, fabrication, or plagiarism. VHA Handbook 1058.02 outlines t
he definitions and processes
for dealing with Research Misconduct. Any other research transgressions are deemed research
impropriety.
The Research Integrity Officer (RIO) is appointed by the Medical Center Director. In many Medical
Centers, the ACOS/R&D is the RIO but individuals even outside of Research Service can serve in that
capacity. The RIO however, should have some background or experience in research.
The RIO should send out a notice to all investigators and staff regarding reporting of allegations of
research misconduct. The RIO receives any allegations of research misconduct and can sequester any
data or other records in order to make an initial determination of whether there is sufficient evidence to
at least on face value, could be construed as research misconduct; this phase is called the Inquiry phase.
Generally, a committee is formed that should include at least a member of the university affiliate if the

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 143 of 149
person alleged to have committed research misconduct is a dual appointee. Working with the affiliate
Research Integrity Officer is helpful in any phase of the research misconduct process. It should also be
determined if inquiry and if needed, investigation into the research misconduct allegation should be led
by the VA or the academic affiliate. With the Inquiry Board, usually a court reporter should be present
to take sworn statements from any witness that would be able to contribute to the Inquiry.
Procurement of court reporter services can best be obtained through Human Resources, Labor and
Employee Relations section. Should the Inquiry yield sufficient information to suggest research
misconduct, then a formal Investigation is convened with an Investigation Committee. The handling of
the Complainant (person making the allegation of research misconduct), the Respondent (person who is
alleged to have committed research misconduct), and witnesses, and other evidence is done under the
aegis of VA Directive 0700 and VA Handbook 0700. Similar to t
he Inquiry, the Investigation also involves
the recording of testimony by a court reporter.
Summary and recommendations made by the Investigation Committee are reviewed by the Medical
Center Director who forwards the report to ORO along with a certificate of completion and concurrence
or non-concurrence of the Investigation Committee report. Essentially ORO performs a procedural
review to be certain that proper procedures and meeting all timelines were adhered to during the
Investigation. If ORO is satisfied that procedural requirements were satisfied, ORO then transmits the
Investigation Report, any exhibits and attachments, and the Medical Center Director’s certificate of
completion to the VISN Director for adjudication. The VISN Director adjudicates the case and renders a
decision regarding either clearing the Respondent of the charges, or applying remediation, or other
dispositions (these can include government-wide debarment for a defined period; prohibition from
conducting VA research for a defined period; removal from a specific research project, or suspension or
termination of an active research award; correction or retraction of published article(s); monitoring or
supervision of future VA research; required validation of data and/or sources; remedial education
and/or mentoring). The VISN Director’s adjudication is sent to ORO and it is ORO that notifies the
respondent as to the outcome of the case. An appeal by the Respondent can be done and the appeal is
made to the Undersecretary for Health. Final agency decision for a filed appeal is issued from the
Undersecretary for Health. In cases where there is no support for the research misconduct allegation,
the reputation of the Respondent must be cleared, and it is expected that the Medical Center leadership
provide assistance in restoring the Respondent’s reputation.

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 144 of 149
Appendix A – VHA DIRECTIVES AND HANDBOOKS and PROGRAM GUIDES
ORD utilizes directives, handbooks and program guides to disseminate policy. These cover a lot of
topics and are subject to change.
To find policies, it is best to start at the ORD website:
https://www.research.va.gov/resources/policies/default.cfm
However, if you know the Directive or Handbook number you can search using the VHA publications
page:
https://www.va.gov/vhapublications/index.cfm

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 145 of 149
Appendix B– Useful Web Links
(As of August 1, 2017)
Description
Web Address
Comment
VA Intranet
Forms
http://vaww.index.va.gov/search/va/index.jsp
Click on VA Forms, VA/VHA
Publication
Research
Resources
https://www.research.va.gov/resources/defau
lt.cfm
(Policies, publications, contact
list, directories, training)
ORD Homepage
(Intranet)
http://vaww.research.va.gov/default.cfm
ORD List of RFA’s
and Program
Announcements
http://vaww.research.va.gov/funding/rfa.cfm
Merit Proposals, etc.
ORD Electronic
submission
information
http://vaww.research.va.gov/funding/electron
ic-submission.cfm
ORPP&E
https://www.research.va.gov/programs/orppe
/default.cfm
VA Central IRB
http://www.research.va.gov/vacentralirb/defa
ult.cfm
The Belmont
Report
http://www.hhs.gov/ohrp/humansubjects/gui
dance/belmont.html
AAALAC
http://www.aaalac.org/
AALAS
http://www.aalas.org/
IPPS
https://vaww.ipps.fsc.va.gov/
Invoice processing system
Concur Travel
Solutions
https://cge.concursolutions.com/
or
VA Identity and Access Management System
(IAM)
VA Travel processing
ePROMISe
http://epromise.research.va.gov/epromise/log
in.htm
eRA Commons
https://commons.era.nih.gov
JIT
https://vaww.gateway.research.va.gov/jit/
PRIM&R
www.primr.org
Advancing ethical standards in
science and research
SRA (Society of
Research
Administrators)
www.srainternational.org
Professional development
organization for research
administrators

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 146 of 149
Appendix C – Research Calendar
(Example: As of August 1, 2017)
Broad category
Discipline
Regulatory item
Point of
Contact
Latest
approval/
Review
Review
Schedule
Regulatory visits
Animal
research
AAALAC
AO/
Veterinarian
Every
3 years
Regulatory visits
Safety
Annual
AWE rounds
AO/
Research Safety
Annually
Regulatory visits
Compliance
ORO RCO
AO/RCO
Regulatory visits
All
ORO
Comprehensive Review
AO/RCO
Every
5 years
External
reports/doc/approvals
Animal
Research
OLAW
Assurances
IACUC
Chair/Veterinari
an
Jun-16
External
reports/doc/approvals
Animal
Research
Semi-Annual
Inspection Report
IACUC Chair/
Veterinarian
June and
Dec
External
reports/doc/approvals
Animal
Research
Annual
USDA Report
Veterinarian/AR
F Supervisor
Every Dec
External
reports/doc/approvals
Animal
Research
VMU
Central Office Report
Veterinarian/AR
F supervisor
Every Dec
External
reports/doc/approvals
Human
Subjects
FWA
IRB
Coordinator
Update
as
Needed
External
reports/doc/approvals
Human
Subjects
IRB
Membership
Listing
IRB
Coordinator
Update
as
Needed
External
Meetings
VISN
Oversight
VISN
Research Roundtable
AO/Administrati
on Assistant
Quarterly
External
Agreements
MOU
VA Central IRB
MOU
R&DC/CIRB
coordinator
Annual
R&DC
May
External
Agreements
MOU
Affiliate IACUC MOU
IACUC/ACOS
Annual
R&DC
May
External
Agreements
MOU
Affiliate Data Security
MOU
ACOS/AO
Annual
R&DC
May
External
Agreements
MOU
Affiliate Safety
MOU
ACOS/AO
Annual
R&DC
May
External
Agreements
MOU
Non-Profit
MOU
PREF ED/ACOS
Annual
R&DC
May
External
Agreements
MOU
Cytometry Core
MOU
AO/ACOS/IFI
Annual
R&DC
May

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 147 of 149
Quarterly review and
R&DC eval
R&D
Quarterly FCOI eval
R&DC
chair/coord/AO
quarterly
Quarterly review and
R&DC eval
R&D
Quarterly Space comm
eval
R&DC
chair/coord/AO
quarterly
Internal document
Human Sub
HRPP IRB SOP
IRB coord
yearly
review
Internal document
R&D
Research MCM
AO
yearly
review
Internal document
Safety
Chemical Hygiene Plan
Research Safety
yearly
review
Internal process
Safety
Hazardous Chemical
inventory + attestation
from PIs
AO
semi-
annual
Internal process
Safety
Semi-annual Bldg XX
walk-through by SRS
SRS Chair/coord
every 6
mon
Internal process
Safety
Annual Emergency and
Disaster Preparedness
Plan
RSO
every
year
Internal process
Safety
Annual Safety/Security
Drills (access, chemical,
security)
Police, Safety
every
year
Internal process
Safety
Quarterly EOC rounds
Research Safety
quarterly
Internal process
Safety
Review of weekly Bldg
XX access
AO
weekly +
monthly
Internal process
Safety
Weekly RSO
walkthrough with
monthly report at SRS
Research Safety
weekly +
monthly
Internal process
Safety
SRS review of Research
Bldg access
AO/SRS coord
semi-
annual
Internal Process
Safety
Annual Review of
off-site VA research
AO/ACOS
Annual
Internal process
Research
program
Performance Evaluation
AO
Internal process
Research
program
Mid-year Performance
Evaluation
AO
Internal process
Research
program
Review submitted and
active funding awards
AO/ACOS/BA
Internal process
Research
program
Review research
instruments
AO/ACOS/BA
Internal process
Research
program
Review off-site research
AO/ACOS
Internal process
Research
program
Review faculty MOU and
effort mapping
AO/ACOS
Internal process
Research
program
Review research budget
AO/ACOS/BA
Internal process
Research
program
Research Equipment
Inventory
AO/ACOS
Equipment
Communications
External
reports/doc/approvals
Grants
SAM registration
AO/BA

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 148 of 149
External
reports/doc/approvals
Grants
RDIS II report
BA
Every
Sept/Oct
External
reports/doc/approvals
Grants
LOI for CSR&D clinical
trial and CDA2
BA
Every Feb
and Aug
External
reports/doc/approvals
Grants
LOI for BLR&D CDA2
BA
Every Feb
and Aug
Funding application
Grants
VA Merit due date for
CSR&D and BLD&D
BA
Every
March
and Sept
Funding application
Grants
LOI for HSR&D & RR&D
VA Merit
BA
Every
May and
Nov
Funding application
Grants
VA Merit due date for
HSR&D and RR&D
BA
Every
June and
Dec
Funding application
Grants
VISN Pilot Funding
Applications
AO/BA
Every Feb
thru May
Funding application
Grants
VA Center of Excellence
Pilot
COIN/COE
Funding application
Grants
ShEEP/LAMB
AO/ACOS

VA ORD – AO/ACOS Guide
ver. 3.0 June 2022, replaces May 2020
Page 149 of 149
Appendix D– Other Commonly Used Forms
Links to Commonly Used Forms and Templates
Form
Name
Link
OF-8
Position Description
https://www.opm.gov/forms/pdf_fill/of8.pdf
OF-69
Assignment Agreement
https://www.opm.gov/forms/pdf_fill/of69.pdf
OF-306
Declaration for Federal
Employment
https://www.opm.gov/forms/pdf_fill/of0306.pdf
7468
Request for Disposition
of Records
http://vaww.va.gov/vaforms/va/pdf/VA7468.pdf
1358
Estimated Miscellaneous
Obligation or Change in
Obligation
http://vaww.va.gov/vaforms/va/pdf/VA1358.pdf
2237
Request, Turn In, and
Receipt for Property or
Services
https://vaww.va.gov/vaforms/va/pdf/VA2237(ES).pdf
OPM 5
CFR 630
Request for Leave or
Approved Absence
https://www.opm.gov/forms/pdf_fill/opm71.pdf
